12 August 2021 As Chemistry Atomic Structure Types Of Orbitals

Atomic Structure | PDF | Atomic Orbital | Atoms
Atomic Structure | PDF | Atomic Orbital | Atoms 12 august 2021 as chemistry atomic structure types of orbitals subshells spdf orbitals 414 views 3 years ago. Learn about atomic orbitals with diagrams of their types, shapes, energy, and a chart of their filling orders.

Atomic Structure | PDF | Atomic Orbital | Atoms
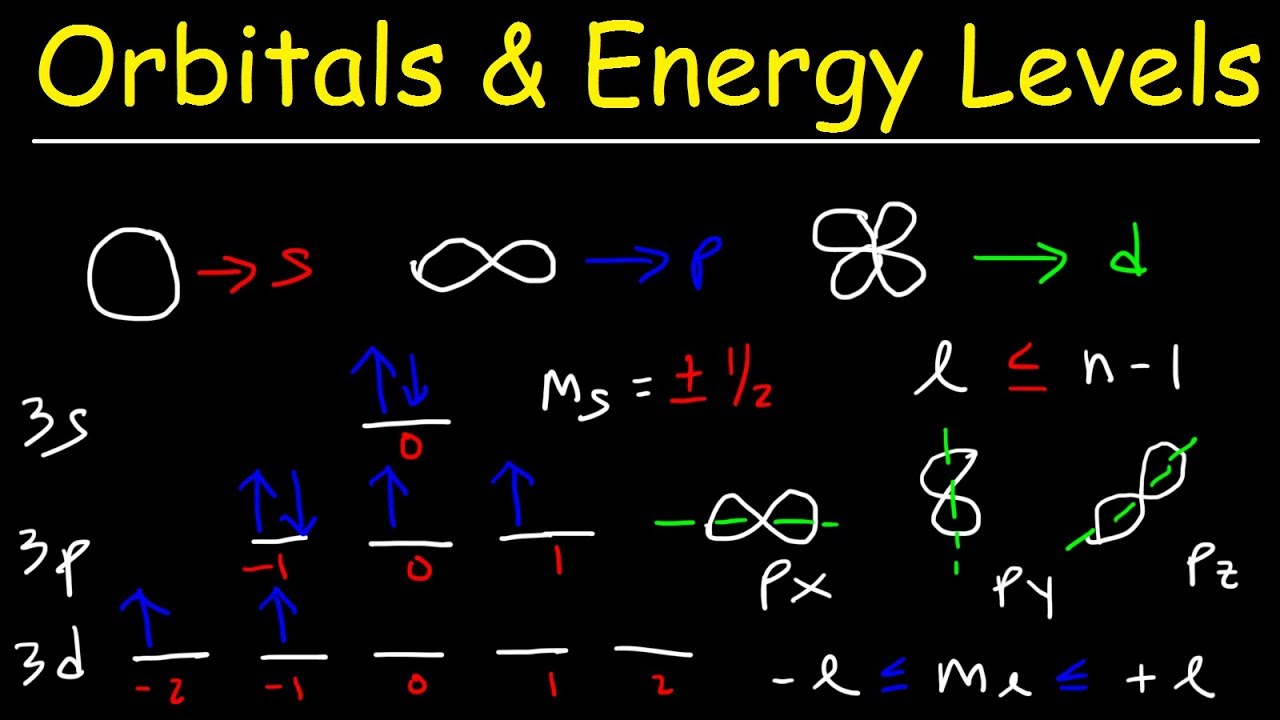
Atomic Structure | PDF | Atomic Orbital | Atoms Their movement is subjected to quantum mechanics, and it is described using 3 dimensional clouds known as orbitals. a total of four different orbitals have been discovered to date. they are the s orbital, p orbital, d orbital, and f orbital. This section explains atomic orbitals, emphasizing their quantum mechanical nature compared to bohr's orbits. it covers the order and energy levels of orbitals from 1s to 3d and details s and p …. Explore atomic orbitals—s, p, d, and f shapes—and how they describe electron behavior in the modern atomic model. includes visuals and key characteristics. In simple terms, atomic orbitals are the regions of space around the nucleus where electrons are most likely to be found. historically, the concept of atomic orbitals emerged from the development of quantum mechanics in the early 20th century.

Atomic Structure | PDF | Atoms | Atomic Orbital
Atomic Structure | PDF | Atoms | Atomic Orbital Explore atomic orbitals—s, p, d, and f shapes—and how they describe electron behavior in the modern atomic model. includes visuals and key characteristics. In simple terms, atomic orbitals are the regions of space around the nucleus where electrons are most likely to be found. historically, the concept of atomic orbitals emerged from the development of quantum mechanics in the early 20th century. A level chemistry revision science section on orbitals. looking at the various shapes of different types of orbitals. In this article, we will learn in detail about atomic orbitals, their names, definition, significance and how they are related to different quantum numbers. what are atomic orbitals? atomic orbitals are spherical paths around the atomic center where the electrons are most likely to be occupied. Orbitals in chemistry are regions within an atom where electrons are most likely to be found. they are defined by quantum mechanics and can be described in terms of their shapes, sizes, and orientations. here's a detailed look at orbitals: an atomic orbital is a region around an atom's nucleus where an electron is likely to be found. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). within each shell of an atom there are some combinations of orbitals.

Atomic Structure-Eng | PDF | Atomic Orbital | Electron Configuration
Atomic Structure-Eng | PDF | Atomic Orbital | Electron Configuration A level chemistry revision science section on orbitals. looking at the various shapes of different types of orbitals. In this article, we will learn in detail about atomic orbitals, their names, definition, significance and how they are related to different quantum numbers. what are atomic orbitals? atomic orbitals are spherical paths around the atomic center where the electrons are most likely to be occupied. Orbitals in chemistry are regions within an atom where electrons are most likely to be found. they are defined by quantum mechanics and can be described in terms of their shapes, sizes, and orientations. here's a detailed look at orbitals: an atomic orbital is a region around an atom's nucleus where an electron is likely to be found. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). within each shell of an atom there are some combinations of orbitals.

Atomic Structure | PDF | Atomic Orbital | Electron
Atomic Structure | PDF | Atomic Orbital | Electron Orbitals in chemistry are regions within an atom where electrons are most likely to be found. they are defined by quantum mechanics and can be described in terms of their shapes, sizes, and orientations. here's a detailed look at orbitals: an atomic orbital is a region around an atom's nucleus where an electron is likely to be found. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). within each shell of an atom there are some combinations of orbitals.
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Related image with 12 august 2021 as chemistry atomic structure types of orbitals
Related image with 12 august 2021 as chemistry atomic structure types of orbitals
About "12 August 2021 As Chemistry Atomic Structure Types Of Orbitals"

















Comments are closed.