Allogeneic Stem Cell Therapy For Acute Ischemic Stroke
Allogeneic Stem Cell Therapy For Acute Ischemic Stroke However, it is unknown whether multistem (hlcm051), a bone marrow derived, allogeneic, multipotent adult progenitor cell product, has the potential to treat ischemic stroke. objective: to assess the efficacy and safety of multistem when administered within 18 to 36 hours of ischemic stroke onset. This study reports findings of the phase 2 3 treatment evaluation of acute stroke using regenerative cells (treasure) trial, which explored multistem safety and efficacy for patients treated within 18 to 36 hours after ischemic stroke onset.

Stem Cell Therapy For Ischemic Stroke Biorender Science Templates Intravenous administration of allogeneic mesenchymal stem cells (mscs) from adipose tissue in patients with acute stroke could be a safe therapy for promoting neurovascular unit repair, consequently supporting better functional recovery. ・the world's first phase 2 3 trial of allogeneic stem cell therapy for stroke. ® within 18‒36 h of ischemic stroke onset was safe. however, no improvement in 90 day outcomes was noted compared to placebo.・no grade 3 or 4 allergic reactions were observ. Multistem ®, an allogeneic “off the shelf” cell therapy, during acute phase of stroke (within the 24 48 hour window) was safe and significantly improved recovery and reduced mortality at one year. Intravenous administration of allo geneic mesenchymal stem cells (mscs) from adipose tissue in patients with acute stroke could be a safe therapy for promoting neurovascular unit repair, consequently supporting better functional recovery.
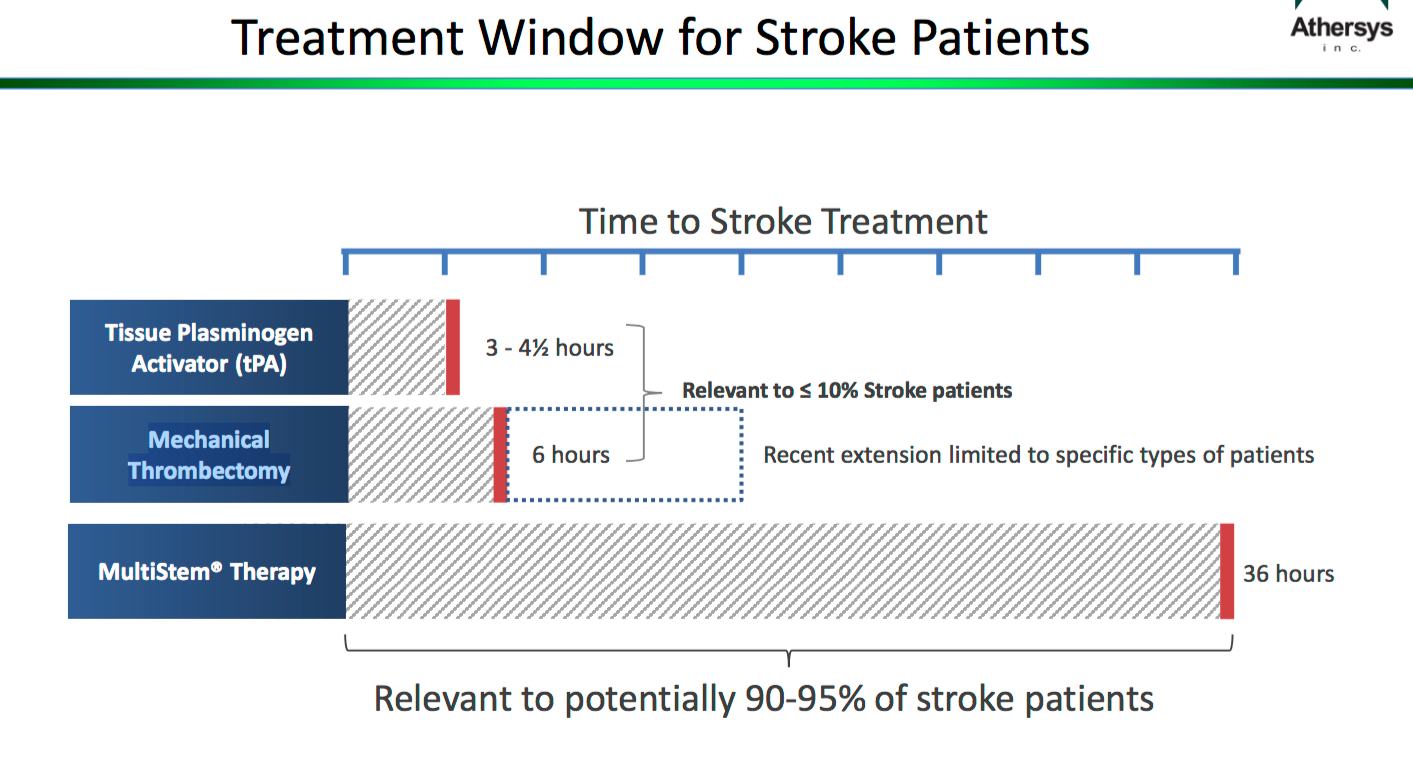
Athersys Stem Cell Therapy For Ischemic Stroke Otcmkts Athxq Multistem ®, an allogeneic “off the shelf” cell therapy, during acute phase of stroke (within the 24 48 hour window) was safe and significantly improved recovery and reduced mortality at one year. Intravenous administration of allo geneic mesenchymal stem cells (mscs) from adipose tissue in patients with acute stroke could be a safe therapy for promoting neurovascular unit repair, consequently supporting better functional recovery. Stem cells represent a promising innovative strategy focused on reduction of neurologic sequelae by enhancement of brain plasticity. we performed a phase iia, randomized, double blind, placebo controlled, single center, pilot clinical trial. Acute ischemic stroke is currently a major cause of disability despite improvement in recanalization therapies. stem cells represent a promising innovative strategy focused on reduction of neurologic sequelae by enhancement of brain plasticity. These authors performed a phase ii, randomized, double blind, placebo controlled, dose escalation trial of an allogeneic adult stem cell, multipotent adult progenitor cells given intravenously 24–48 hours after stroke onset. Intravenous administration of allogeneic mesenchymal stem cells (mscs) from adipose tissue in patients with acute stroke could be a safe therapy for promoting neurovascular unit repair, consequently supporting better functional recovery.

Comments are closed.