Blood Based Test Offers Effective Screening Option For Colorectal Cancer
:max_bytes(150000):strip_icc()/VWH-GettyImages-1343991775-4d54cae09701441f9bbcdd90c52a38be.jpg)
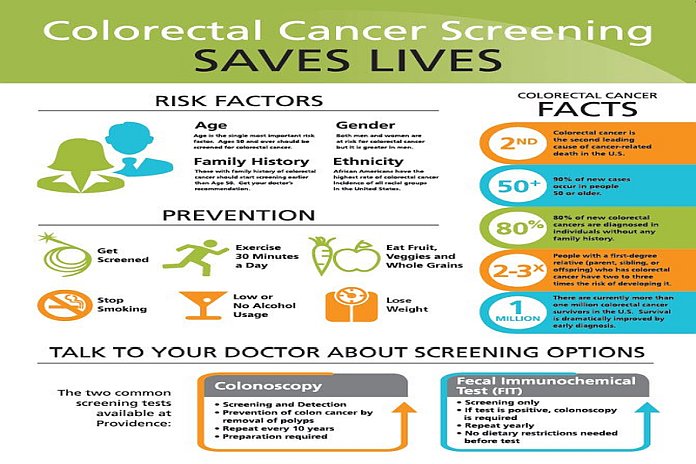
A New Blood Test To Screen For Colorectal Cancer Shows Promise Fda approved the first blood test for the primary screening of colorectal cancer. where does it fit in with options like colonoscopy and stool tests?. A blood based test has the potential to improve screening adherence, detect colorectal cancer earlier, and reduce colorectal cancer–related mortality. we assessed the performance.

Blood Test May Provide New Option For Colorectal Cancer Screening Blood tests for colorectal cancer are an option for patients who would otherwise not be screened, but they are not as effective as colonoscopies or stool tests, a stanford medicine led study found. The discovery of circulating and cell free tumour dna in the blood has ushered in new possibilities for blood based colorectal cancer screening. 1 the septin 9 test is currently the only us federal drug administration approved blood based colorectal cancer screening test that is available; however, due to its low sensitivity, this test was not. All of the currently available crc screening test options fall short of this ideal in at least 1 way, limiting their effectiveness. thus, there is an ongoing search for more agreeable screening test options. The fda has approved a new blood test for colorectal cancer, offering an alternative to colonoscopies and stool tests and potentially boosting screening rates for this deadly disease.

Fda Approves Blood Test For Colorectal Cancer Screening Harvard Health All of the currently available crc screening test options fall short of this ideal in at least 1 way, limiting their effectiveness. thus, there is an ongoing search for more agreeable screening test options. The fda has approved a new blood test for colorectal cancer, offering an alternative to colonoscopies and stool tests and potentially boosting screening rates for this deadly disease. Freenome blood based crc screening test met all primary endpoints demonstrating 79.2% sensitivity for crc, 91.5% specificity for acn, 90.8% negative predictive value (npv) for acn and 15.5% positive predictive value (ppv) for acn. On july 29, 2024, the food and drug administration (fda) approved a blood test that can help with primary colorectal cancer screening. the test is guardant health’s shield blood test, and the. Blood based screening tests offer a non invasive, more convenient alternative for patients not amenable to the preferred colonoscopy or stool based screening tests. Blood based testing offers a promising complementary approach that may boost patient adherence among unscreened individuals, she added. the study evaluated the clinical performance of the.
Innovative Blood Test Shows Early Promise For Effective Colorectal Freenome blood based crc screening test met all primary endpoints demonstrating 79.2% sensitivity for crc, 91.5% specificity for acn, 90.8% negative predictive value (npv) for acn and 15.5% positive predictive value (ppv) for acn. On july 29, 2024, the food and drug administration (fda) approved a blood test that can help with primary colorectal cancer screening. the test is guardant health’s shield blood test, and the. Blood based screening tests offer a non invasive, more convenient alternative for patients not amenable to the preferred colonoscopy or stool based screening tests. Blood based testing offers a promising complementary approach that may boost patient adherence among unscreened individuals, she added. the study evaluated the clinical performance of the.
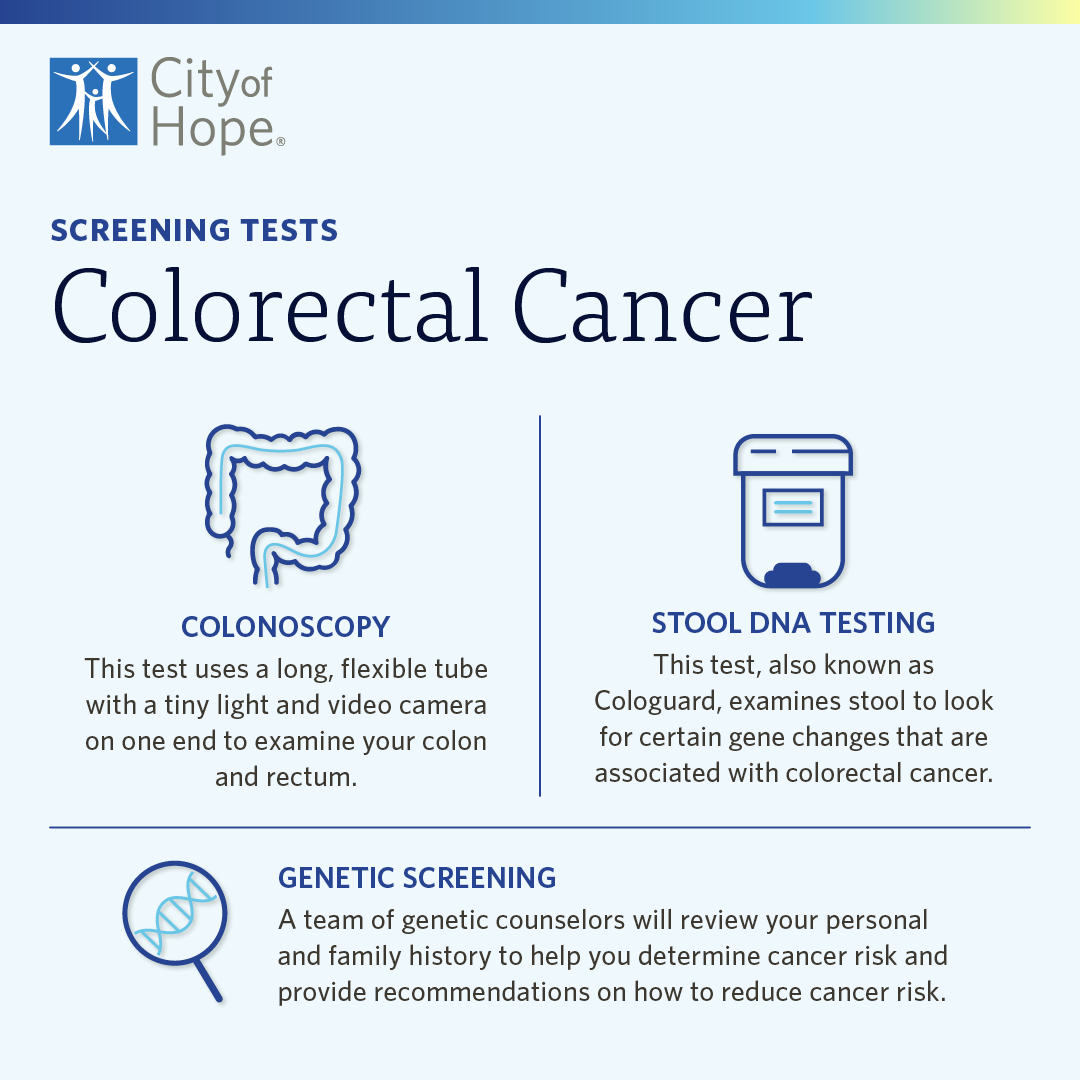
Colorectal Cancer Screening The Test That Can Save Your Life City Of Blood based screening tests offer a non invasive, more convenient alternative for patients not amenable to the preferred colonoscopy or stool based screening tests. Blood based testing offers a promising complementary approach that may boost patient adherence among unscreened individuals, she added. the study evaluated the clinical performance of the.

Comments are closed.