Cybersecurity For Medical Devices Omc Medical

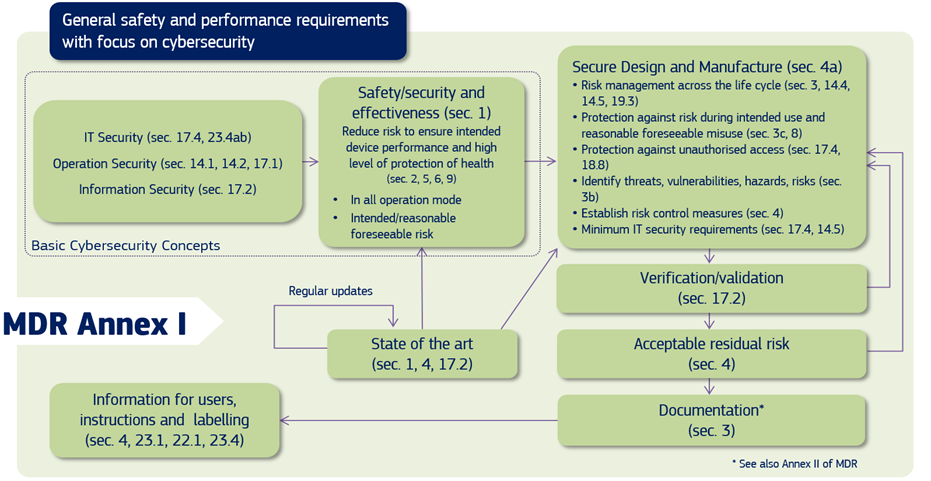
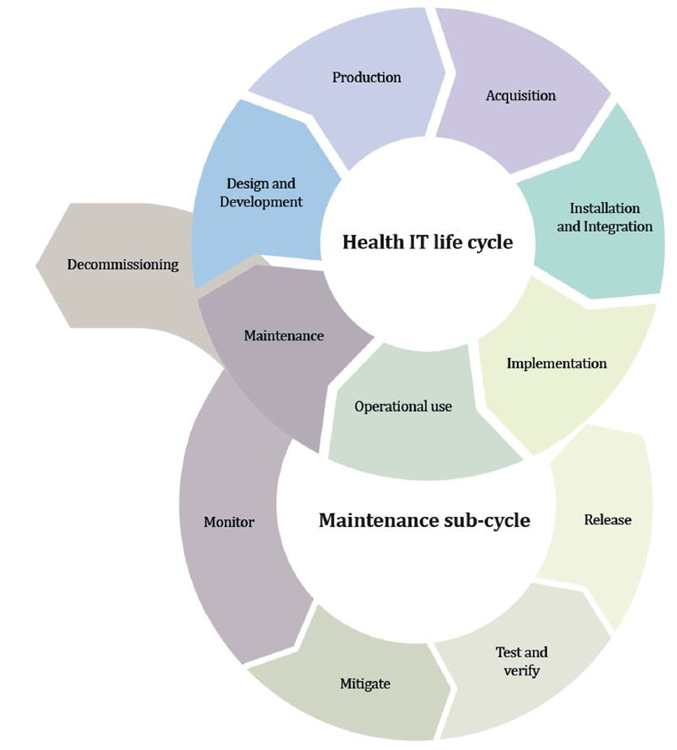
Cybersecurity For Medical Devices Who S Accountable Eqms Assurx The fda alerts all medical device users and manufacturers about a cybersecurity vulnerability identified for the axeda agent and axeda desktop server. the agent and desktop server of axeda are used in many medical devices across several medical device manufacturers, and all the versions of the axeda agent and axeda desktop server are affected. In the eu, both the mdr and ivdr annex i create requirements for mandate consideration of medical device cybersecurity, and the medical device coordination group (mdcg), in its guidance, explains to the manufacturers of medical devices how to fulfil all the relevant essential requirements regarding cybersecurity.

Cybersecurity Of Medical Devices Pdf Eu cybersecurity laws for medical devices are advancing, and the use of software medical devices is also increasing daily. the increased interconnection of medical devices to computer networks and technological convergence have made devices and software programmes vulnerable to mishaps. The role cybersecurity and device experts play in the review of medical devices. given the job cuts being made at the department of health and human services, representatives asked fu to explain the role cybersecurity experts play in the review of medical devices, and how cuts to the fda could impact that work. When submitting a medical device for fda approval—whether it’s a 510 (k), de novo, or pma—manufacturers must now provide comprehensive documentation demonstrating their device’s. With the rise of iot and cloud based solutions, medical device cybersecurity is facing unprecedented threats. cybercriminals exploit vulnerabilities in medical devices, putting patient safety at risk.

Eu Cybersecurity Laws For Medical Devices Omc Medical When submitting a medical device for fda approval—whether it’s a 510 (k), de novo, or pma—manufacturers must now provide comprehensive documentation demonstrating their device’s. With the rise of iot and cloud based solutions, medical device cybersecurity is facing unprecedented threats. cybercriminals exploit vulnerabilities in medical devices, putting patient safety at risk. Cybersecurity is a shared responsibility across the medical device ecosystem, and navigating it effectively requires experience, strategic foresight, and cross functional expertise. at clarimed, we help companies design and deliver safer, smarter, and more resilient digital health technologies without compromising speed or compliance. Increasing medical device sophistication relates to the inclusion of more connectivity and advanced features like artificial intelligence (ai) integration and means that the attack surface for cybercriminals is continuing to expand. This three day seminar focuses on the dynamic cybersecurity landscape within the medical device industry including standards and regulatory compliance. the curriculum covers the fundamental principles of iec 62304, the international standard for medical device software. A recent amendment to the federal food, drug, and cosmetic act (fd&c act) added a new section about medical device cybersecurity for “cyber devices.” if a device uses software that connects to the internet, it is most likely considered a cyber device and subject to the new 524b section of the fd& c act. this provision has been effective.
Cybersecurity For Medical Devices Omc Medical Cybersecurity is a shared responsibility across the medical device ecosystem, and navigating it effectively requires experience, strategic foresight, and cross functional expertise. at clarimed, we help companies design and deliver safer, smarter, and more resilient digital health technologies without compromising speed or compliance. Increasing medical device sophistication relates to the inclusion of more connectivity and advanced features like artificial intelligence (ai) integration and means that the attack surface for cybercriminals is continuing to expand. This three day seminar focuses on the dynamic cybersecurity landscape within the medical device industry including standards and regulatory compliance. the curriculum covers the fundamental principles of iec 62304, the international standard for medical device software. A recent amendment to the federal food, drug, and cosmetic act (fd&c act) added a new section about medical device cybersecurity for “cyber devices.” if a device uses software that connects to the internet, it is most likely considered a cyber device and subject to the new 524b section of the fd& c act. this provision has been effective.
Cybersecurity For Medical Devices Omc Medical This three day seminar focuses on the dynamic cybersecurity landscape within the medical device industry including standards and regulatory compliance. the curriculum covers the fundamental principles of iec 62304, the international standard for medical device software. A recent amendment to the federal food, drug, and cosmetic act (fd&c act) added a new section about medical device cybersecurity for “cyber devices.” if a device uses software that connects to the internet, it is most likely considered a cyber device and subject to the new 524b section of the fd& c act. this provision has been effective.
Cybersecurity For Medical Devices Omc Medical

Comments are closed.