Dr Gomella On The Fda Approval Of Enzalutamide In Nonmetastatic Crpc
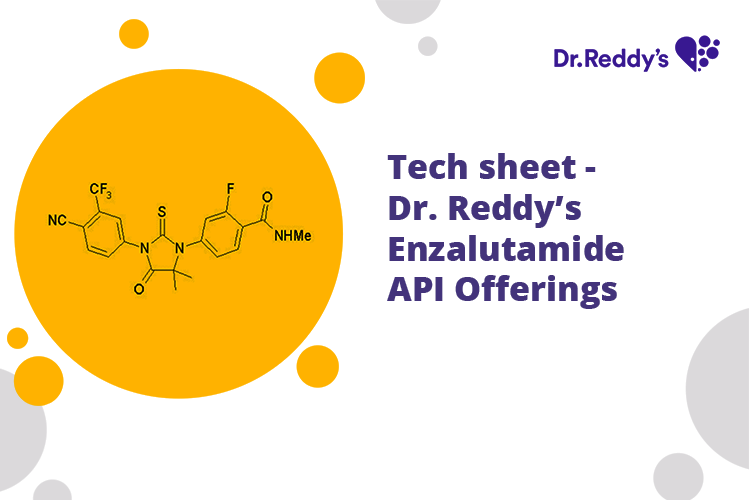
Enzalutamide Api Manufacturer And Supplier Cas 915087 33 1 Dr Reddy S Leonard gomella, md, professor, chair, department of urology, director, sidney kimmel cancer center network, thomas jefferson university hospital, discusses the fda approval of enzalutamide. Leonard gomella, md, professor, chair, department of urology, director, sidney kimmel cancer center network, thomas jefferson university hospital, discusses.

Dr Reddy Lab Enzalutamide40mg Capsules At 19000 Box In New Delhi On november 16, 2023, the food and drug administration approved enzalutamide (xtandi, astellas pharma us, inc.) for non metastatic castration sensitive prostate cancer (nmcspc) with. Leonard gomella, md, professor, chair, department of urology, director, sidney kimmel cancer center network, thomas jefferson university hospital, discusses enzalutamide (xtandi) for patients. This latest approval now makes enzalutamide the only fda approved oral medication indicated for both metastatic and nonmetastatic castration resistant prostate cancer. The food and drug administration (fda) also approved sipuleucel t, an autologous cellular immunotherapy, for the treatment of asymptomatic or mild symptomatic castration resistant prostate cancer (crpc) patients. 11.
.jpg)
Avalon Pharma Exporter Supplier Of Generic Medicines Exporter This latest approval now makes enzalutamide the only fda approved oral medication indicated for both metastatic and nonmetastatic castration resistant prostate cancer. The food and drug administration (fda) also approved sipuleucel t, an autologous cellular immunotherapy, for the treatment of asymptomatic or mild symptomatic castration resistant prostate cancer (crpc) patients. 11. The approval makes enzalutamide the first and only androgen receptor signaling inhibitor approved for the treatment of nonmetastatic castration sensitive prostate cancer with biochemical recurrence at high risk for metastatic. “the fda has approved enzalutamide (xtandi) for the treatment of patients with nonmetastatic castration resistant prostate cancer (crpc), according to pfizer and astellas, the codevelopers of the antiandrogen agent. The current approval was based on a statistically significant improvement in metastasis free survival for patients receiving enzalutamide in the phase 3 prosper trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic crpc to 160 mg of oral enzalutamide daily or to placebo. The fda initially approved enzalutamide in 2012 as a treatment for men with metastatic crpc following docetaxel. this approval was expanded in 2014 to include treatment with the antiandrogen agent prior to chemotherapy.
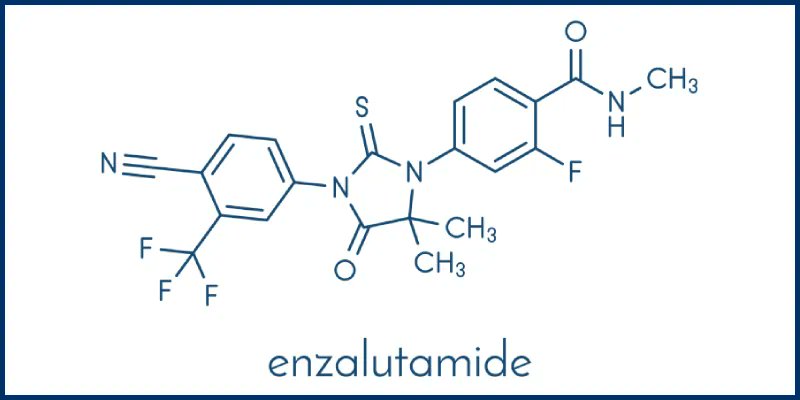
Gu Oncology Now On Twitter New Research From The Enzamet Trial Has The approval makes enzalutamide the first and only androgen receptor signaling inhibitor approved for the treatment of nonmetastatic castration sensitive prostate cancer with biochemical recurrence at high risk for metastatic. “the fda has approved enzalutamide (xtandi) for the treatment of patients with nonmetastatic castration resistant prostate cancer (crpc), according to pfizer and astellas, the codevelopers of the antiandrogen agent. The current approval was based on a statistically significant improvement in metastasis free survival for patients receiving enzalutamide in the phase 3 prosper trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic crpc to 160 mg of oral enzalutamide daily or to placebo. The fda initially approved enzalutamide in 2012 as a treatment for men with metastatic crpc following docetaxel. this approval was expanded in 2014 to include treatment with the antiandrogen agent prior to chemotherapy.
Fda Expands Enzalutamide Approval To Earlier Prostate Cancer The current approval was based on a statistically significant improvement in metastasis free survival for patients receiving enzalutamide in the phase 3 prosper trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic crpc to 160 mg of oral enzalutamide daily or to placebo. The fda initially approved enzalutamide in 2012 as a treatment for men with metastatic crpc following docetaxel. this approval was expanded in 2014 to include treatment with the antiandrogen agent prior to chemotherapy.

Identifying And Targeting Enzalutamide Resistance Mechanisms In

Comments are closed.