Examples Of Common Functional Groups In Organic Chemistry

Common Functional Groups In Organic Chemistry Organic Chemistry Functional groups are specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule. common examples of functional groups are alcohols, alkenes, alkynes, amines, carboxylic acids, aldehydes, ketones, esters, and ethers, among others. Functional groups undergo the same chemical reactions no matter how large or small the molecule is. covalent bonds link the atoms within functional groups and connect them to the rest of the molecule. examples of functional groups include the hydroxyl group, ketone group, amine group, and ether group.
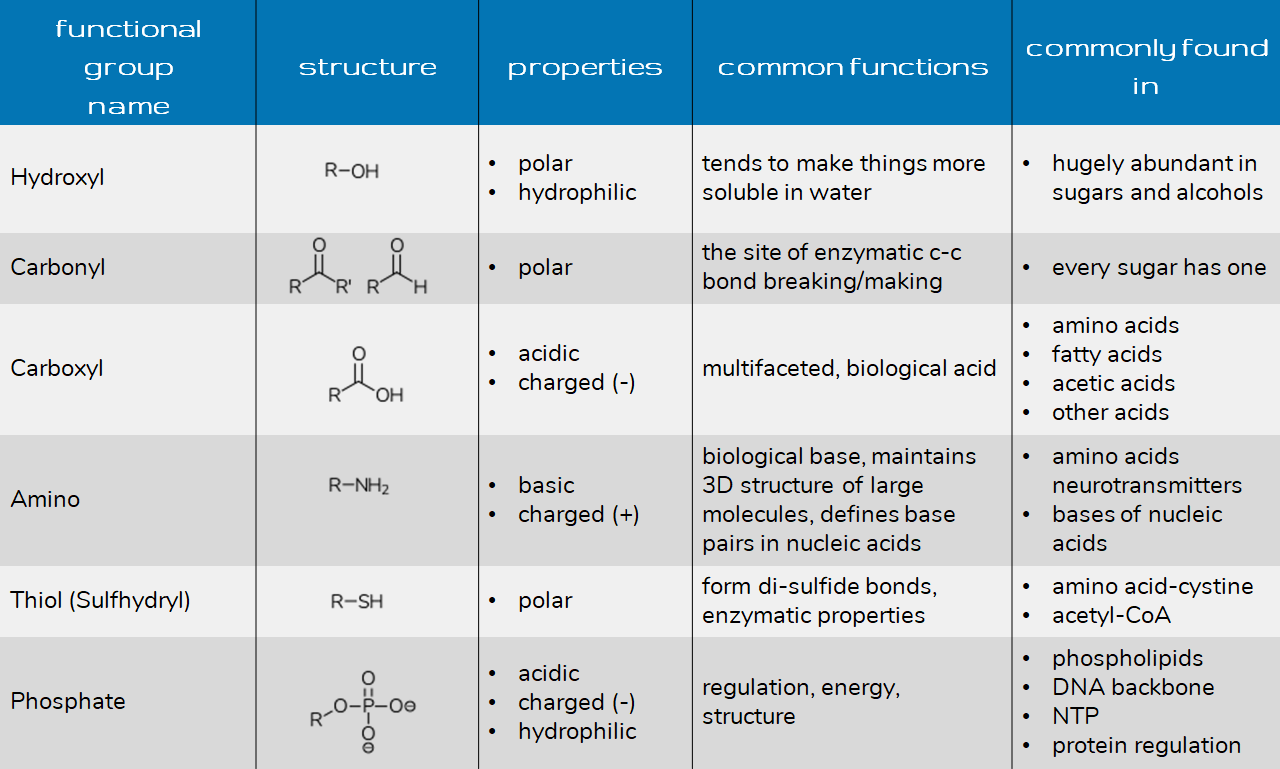
Examples Of Common Functional Groups In Organic Chemistry Nitrogen containing functional groups. the main groups with nitrogen are amines, amides, and nitriles. other groups, such as imines, azos, and azides are important but come up less. sulfur containing functional groups. thiols, thioethers, and disulfides are the most common functional groups with sulfur. to remember the sulfur groups, notice the. A functional group in organic chemistry is a collection of atoms within molecules which bind together to react in predictable ways. examples of functional groups include the group hydroxyl, ketone, amine, and ether. We define a functional group as a specific combination of atoms that has a unique set of chemical properties. thus, recognizing functional groups in a molecule will get you one step closer to identifying possible reactions and transformations you may have for that molecule. let’s look at an example above. For each of the functional groups listed below a single example is shown on the right. the examples are all of molecules with carbon chains of 3 carbon atoms, hence the names include " prop " as part of the name, e.g. propane, propene, etc where available links to pages of more examples of chemicals of the same type are included.
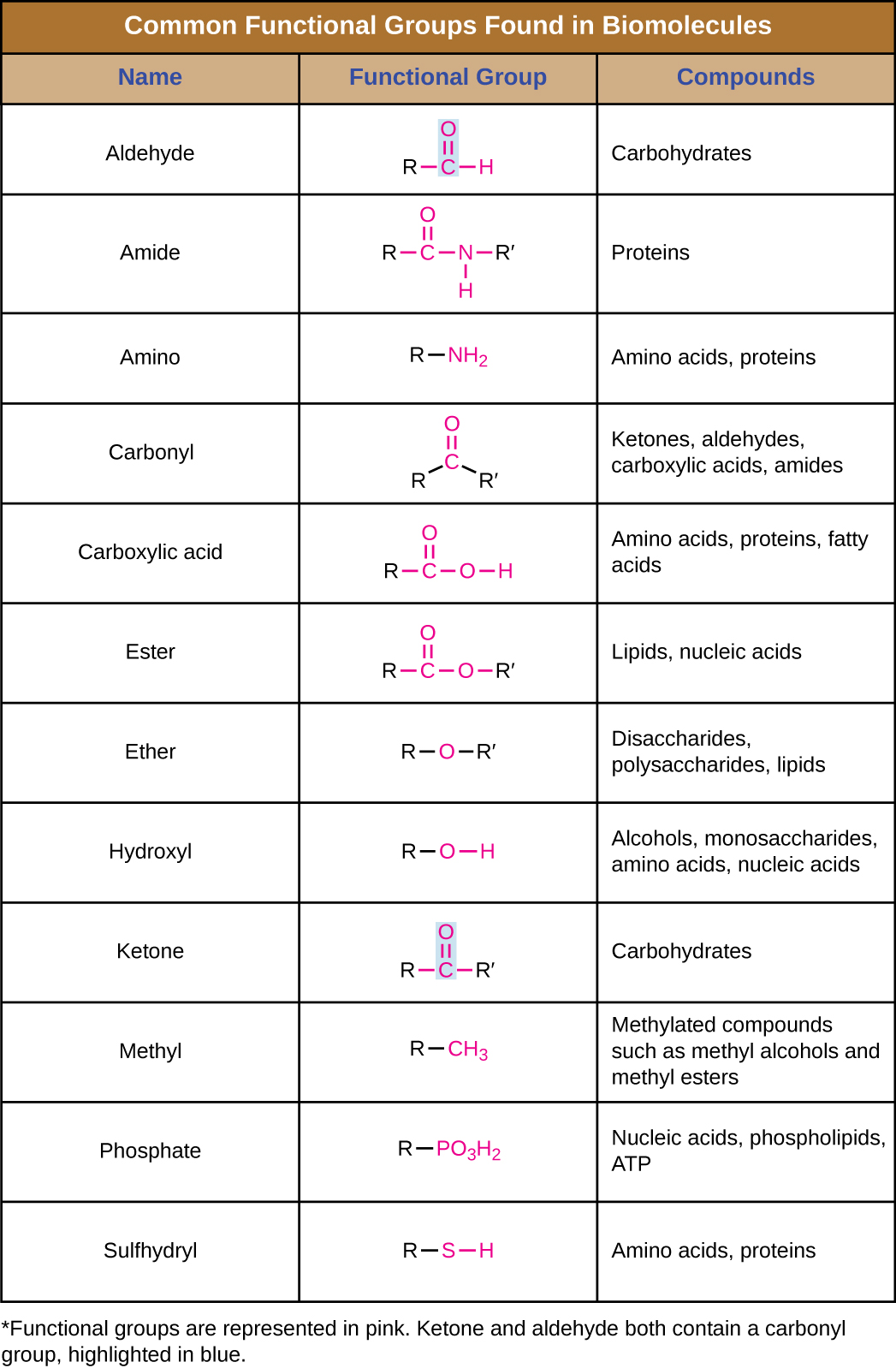
Examples Of Common Functional Groups In Organic Chemistry We define a functional group as a specific combination of atoms that has a unique set of chemical properties. thus, recognizing functional groups in a molecule will get you one step closer to identifying possible reactions and transformations you may have for that molecule. let’s look at an example above. For each of the functional groups listed below a single example is shown on the right. the examples are all of molecules with carbon chains of 3 carbon atoms, hence the names include " prop " as part of the name, e.g. propane, propene, etc where available links to pages of more examples of chemicals of the same type are included. Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. the structure of capsaicin, the compound responsible for the heat in peppers, incorporates several functional groups, labeled in the figure below and explained throughout this section. In organic chemistry, the most common functional groups are carbonyls (c=o), alcohols ( oh), carboxylic acids (co 2 h), esters (co 2 r), and amines (nh 2). it is important to be able to recognize the functional groups and the physical and chemical properties that they afford compounds. organic chemistry functional groups made easy and memorizable!. Some of the most common functional groups are presented in the following sections. organic molecules vary greatly in size and when focusing on functional groups, we want to direct our attention to the atoms involved in the functional group. as a result, the abbreviation r is used in some examples. Look at table 3.1, which lists many of the common functional groups and gives simple examples of their occurrence. some functional groups have only carbon–carbon double or triple bonds; others have halogen atoms; and still others contain oxygen, nitrogen, or sulfur. much of the chemistry you’ll be studying is the chemistry of these.
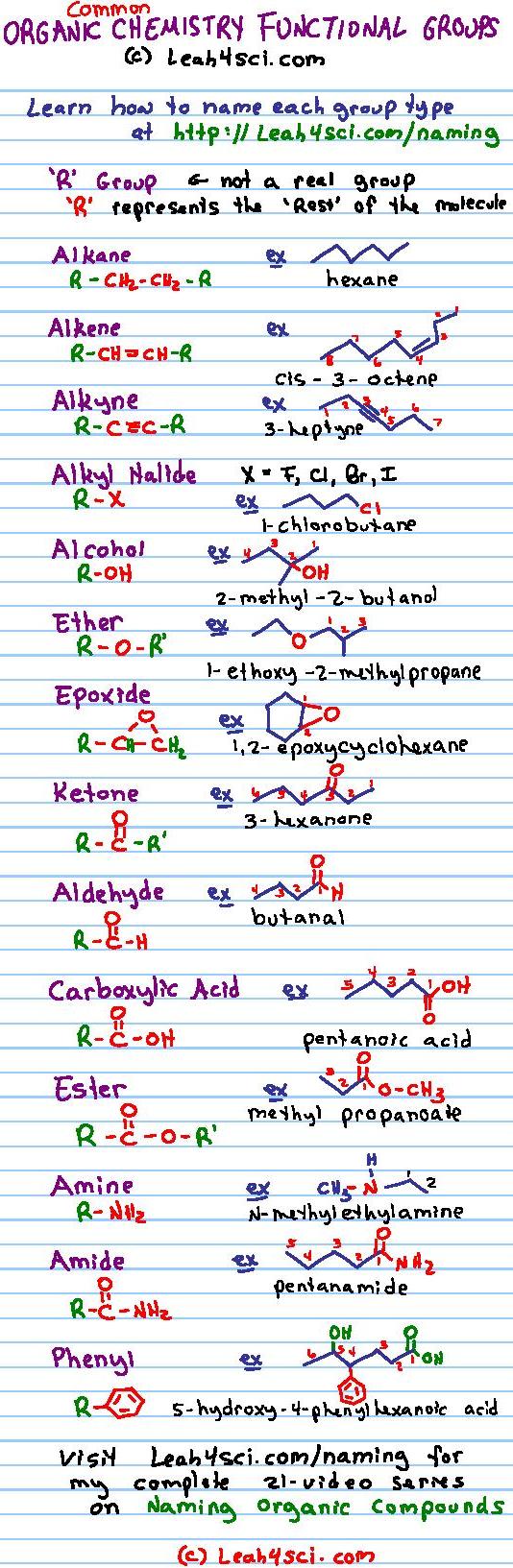
Examples Of Common Functional Groups In Organic Chemistry Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. the structure of capsaicin, the compound responsible for the heat in peppers, incorporates several functional groups, labeled in the figure below and explained throughout this section. In organic chemistry, the most common functional groups are carbonyls (c=o), alcohols ( oh), carboxylic acids (co 2 h), esters (co 2 r), and amines (nh 2). it is important to be able to recognize the functional groups and the physical and chemical properties that they afford compounds. organic chemistry functional groups made easy and memorizable!. Some of the most common functional groups are presented in the following sections. organic molecules vary greatly in size and when focusing on functional groups, we want to direct our attention to the atoms involved in the functional group. as a result, the abbreviation r is used in some examples. Look at table 3.1, which lists many of the common functional groups and gives simple examples of their occurrence. some functional groups have only carbon–carbon double or triple bonds; others have halogen atoms; and still others contain oxygen, nitrogen, or sulfur. much of the chemistry you’ll be studying is the chemistry of these.
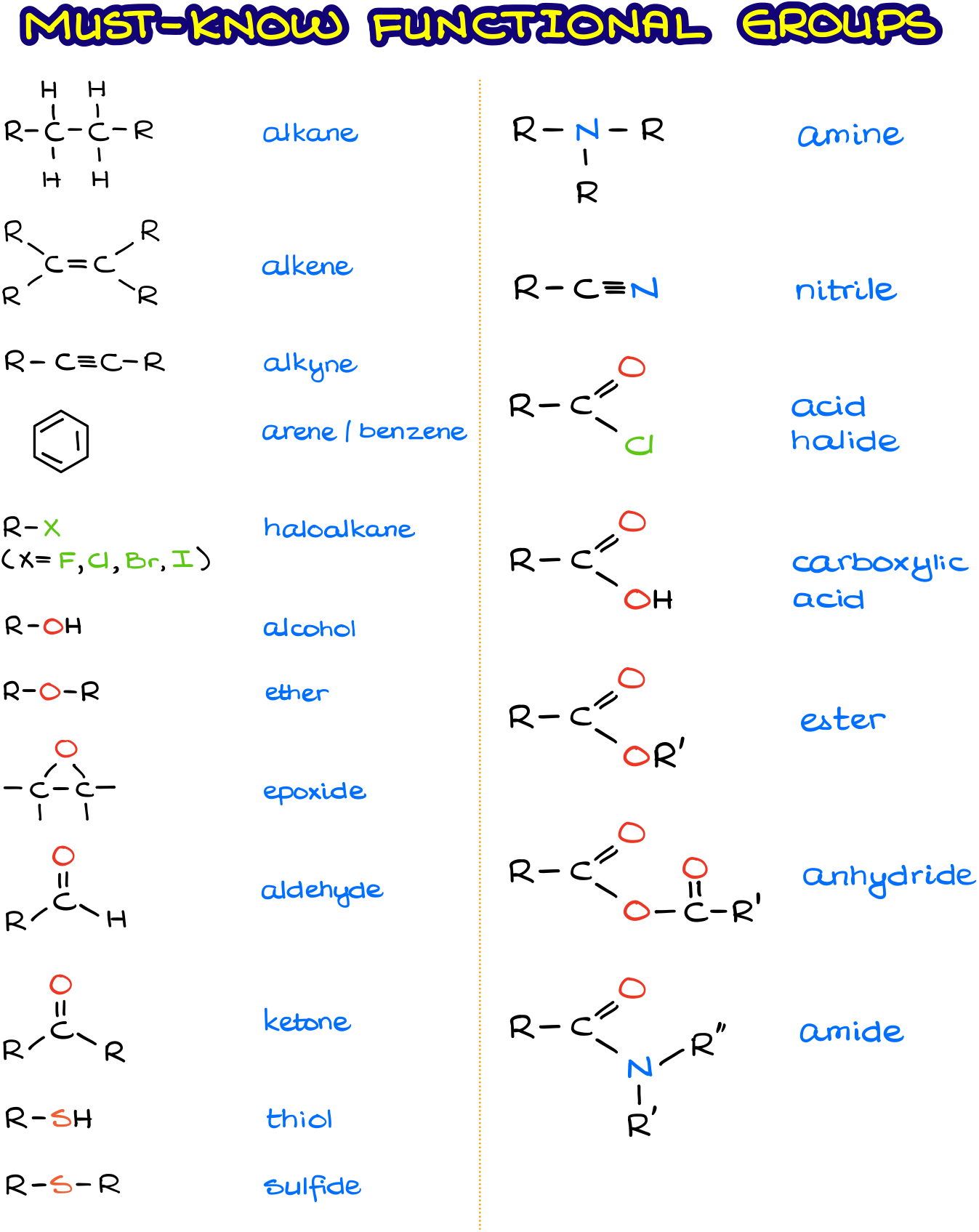
Examples Of Common Functional Groups In Organic Chemistry Some of the most common functional groups are presented in the following sections. organic molecules vary greatly in size and when focusing on functional groups, we want to direct our attention to the atoms involved in the functional group. as a result, the abbreviation r is used in some examples. Look at table 3.1, which lists many of the common functional groups and gives simple examples of their occurrence. some functional groups have only carbon–carbon double or triple bonds; others have halogen atoms; and still others contain oxygen, nitrogen, or sulfur. much of the chemistry you’ll be studying is the chemistry of these.

Examples Of Common Functional Groups In Organic Chemistry

Comments are closed.