Fda Approves First Over The Counter Naloxone Nasal Spray
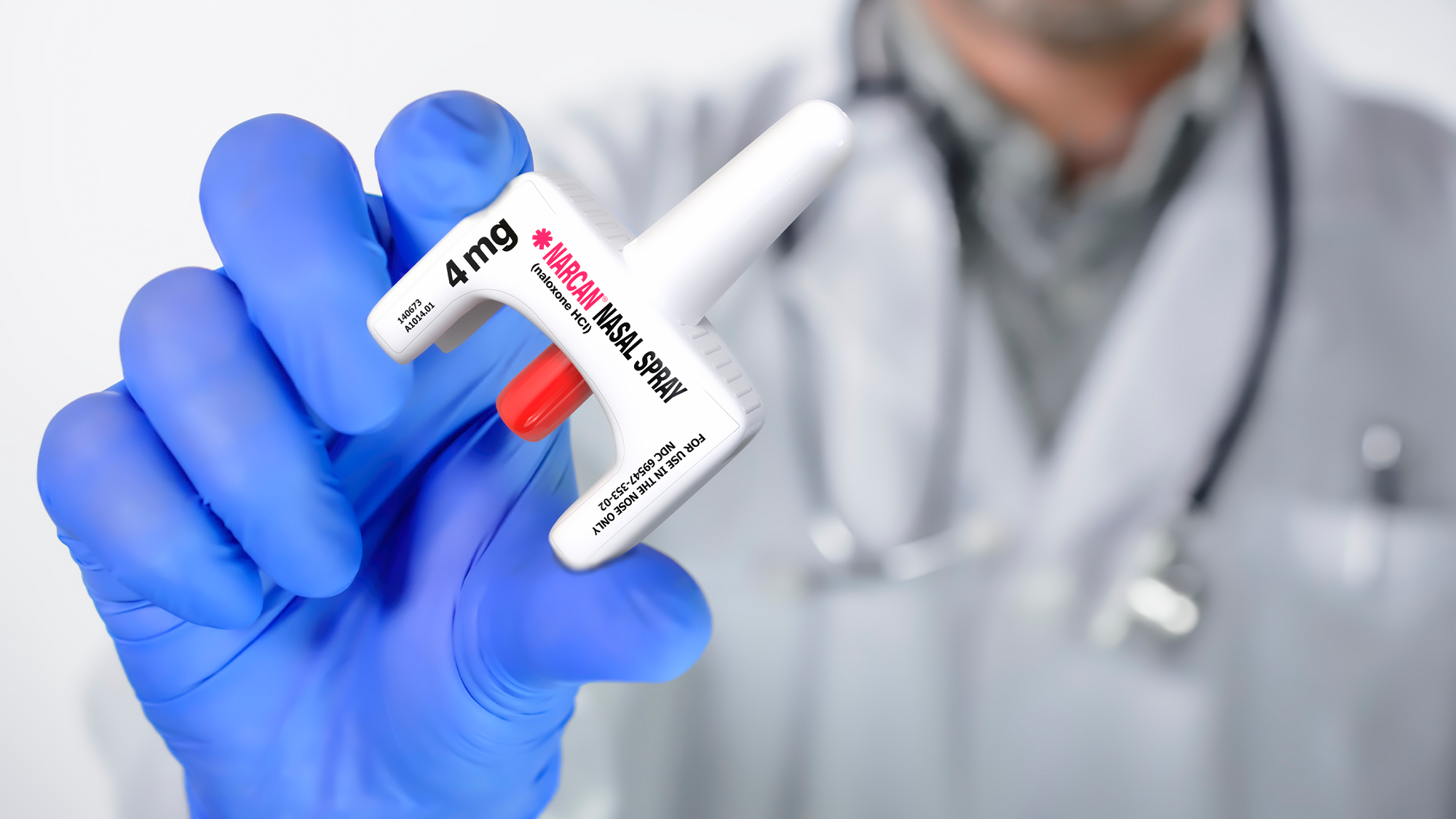
Fda Approves First Over The Counter Naloxone Nasal Spray Rutgers Today, the u.s. food and drug administration approved narcan, 4 milligram (mg) naloxone hydrochloride nasal spray for over the counter (otc), nonprescription, use – the first naloxone product. In march 2023, the first otc naloxone hydrochloride 4 mg (narcan; emergent biosolutions) was approved for use without a prescription. this followed the decision of 2 fda panels that unanimously voted to recommend that naloxone be made available without a prescription being required.

Fda Approves First Over The Counter Naloxone Nasal Spray Pacific The u.s. food and drug administration (fda) on march 29 approved narcan (naloxone) 4 mg hydrochloride nasal spray for purchase without a prescription. narcan rapidly reverses the effects of an opioid overdose. The us food and drug administration (fda) approved the first over the counter (otc) naloxone product for the emergency treatment of opioid overdose. the fda announced the approval of naloxone hydrochloride nasal spray 4 mg (narcan®, emergent biosolutions) in a march 29, 2023, press release. The fda approved the first over the counter narcan (naloxone) product on march 29. Silver spring, md., march 29, 2023 prnewswire today, the u.s. food and drug administration approved narcan, 4 milligram (mg) naloxone hydrochloride nasal spray for over the counter (otc),.

Fda Approves First Over The Counter Naloxone Nasal Spray Dfc The fda approved the first over the counter narcan (naloxone) product on march 29. Silver spring, md., march 29, 2023 prnewswire today, the u.s. food and drug administration approved narcan, 4 milligram (mg) naloxone hydrochloride nasal spray for over the counter (otc),. Be ready – at home or on the go. narcan ® nasal spray is a treatment designed to rapidly reverse the effects of a life threatening opioid emergency. available as an over the counter medicine. be prepared with narcan® nasal spray naloxone, the active ingredient in narcan®nasal spray, competes with opioids to bind with the same receptors in the brain, reversing the effects of an opioid. About narcan® nasal spray narcan ® naloxone hcl nasal spray 4 mg is the first fda approved, over the counter (otc) 4 mg naloxone product for the emergency treatment of opioid overdose. — a new study from rti international, an independent scientific research institute, shows that over the counter (otc) sales of the opioid reversal medication naloxone remain limited in areas hardest hit by opioid overdoses, despite the u.s. food and drug administration’s march 2023 approval of the nasal spray for nonprescription use. The us food & drug administration (fda) announced that it has approved narcan for nonprescription, over the counter (otc) use. narcan, 4 mg naloxone hydrochloride nasal spray, is the first naloxone product to receive fda approval for nonprescription use.

Fda Approves First Over The Counter Naloxone Nasal Spray Be ready – at home or on the go. narcan ® nasal spray is a treatment designed to rapidly reverse the effects of a life threatening opioid emergency. available as an over the counter medicine. be prepared with narcan® nasal spray naloxone, the active ingredient in narcan®nasal spray, competes with opioids to bind with the same receptors in the brain, reversing the effects of an opioid. About narcan® nasal spray narcan ® naloxone hcl nasal spray 4 mg is the first fda approved, over the counter (otc) 4 mg naloxone product for the emergency treatment of opioid overdose. — a new study from rti international, an independent scientific research institute, shows that over the counter (otc) sales of the opioid reversal medication naloxone remain limited in areas hardest hit by opioid overdoses, despite the u.s. food and drug administration’s march 2023 approval of the nasal spray for nonprescription use. The us food & drug administration (fda) announced that it has approved narcan for nonprescription, over the counter (otc) use. narcan, 4 mg naloxone hydrochloride nasal spray, is the first naloxone product to receive fda approval for nonprescription use.

Comments are closed.