Fda Requirements For Medical Devices Prioritize Cybersecurity To Avoid

Fda Requirements For Medical Devices Prioritize Cybersecurity To Avoid Understanding the components of the food and drug administration (fda) guidance and their legal ramifications is crucial as the agency sets out to implement its new medical device cybersecurity protocols and prepares for strict enforcement in october 2023. healthcare stakeholders must bring into their infrastructure cybersecurity provisions. A: the 2023 guidance cybersecurity in medical devices: quality system considerations and content of premarket submissions provides recommendations on cybersecurity considerations for.

Cybersecurity In Medical Devices Today S Medical Developments Our topic today is the final guidance titled, cybersecurity in medical devices: quality system considerations and content of premarket submissions, which was issued on september 27, 2023 . Increased scrutiny over the vulnerabilities of medical devices forced the fda to rethink its cybersecurity requirements. the fbi issued a report, finding that 53% of digital medical devices and internet connected products had critical vulnerabilities. unpatched and outdated devices were the key weak link. Key fda cybersecurity requirements the fda’s guidance highlights several critical areas that manufacturers must address to ensure cybersecurity compliance. these form the backbone of the secure product development framework (spdf), which is now essential for every medical device manufacturer. 1. secure product development framework (spdf). The fda cybersecurity requirements for medical “cyber devices” went into effect on oct. 1, 2023. the fda has made it clear that it cannot even accept submissions that don’t contain the cybersecurity requirements. this article shares 4 steps to navigate the new requirements.

New Cybersecurity Guidelines For Medical Devices Tackle Evolving Threats Key fda cybersecurity requirements the fda’s guidance highlights several critical areas that manufacturers must address to ensure cybersecurity compliance. these form the backbone of the secure product development framework (spdf), which is now essential for every medical device manufacturer. 1. secure product development framework (spdf). The fda cybersecurity requirements for medical “cyber devices” went into effect on oct. 1, 2023. the fda has made it clear that it cannot even accept submissions that don’t contain the cybersecurity requirements. this article shares 4 steps to navigate the new requirements. Fda cybersecurity for medical devices demands that manufacturers prioritize security from design through deployment, and even beyond. incorporating medical device penetration testing, proactive threat management, and postmarket security measures, manufacturers can ensure safety for patients as well as guarantee fda compliance, and preserve. These recommendations are intended to promote consistency, facilitate efficient premarket review, and help ensure that marketed medical devices are sufficiently resilient to cybersecurity. The u.s. food and drug administration (fda) has published new guidance on its requirement for medical device manufacturers to include details of the cybersecurity measures that have been implemented for new products in premarket submissions. As part of its secure product development framework (spdf), the fda recommends the use of the following techniques and tools to help identify and mitigate cybersecurity risks in medical devices. 1. threat modeling. threat modeling aids in identifying and addressing potential cybersecurity threats within medical device systems.
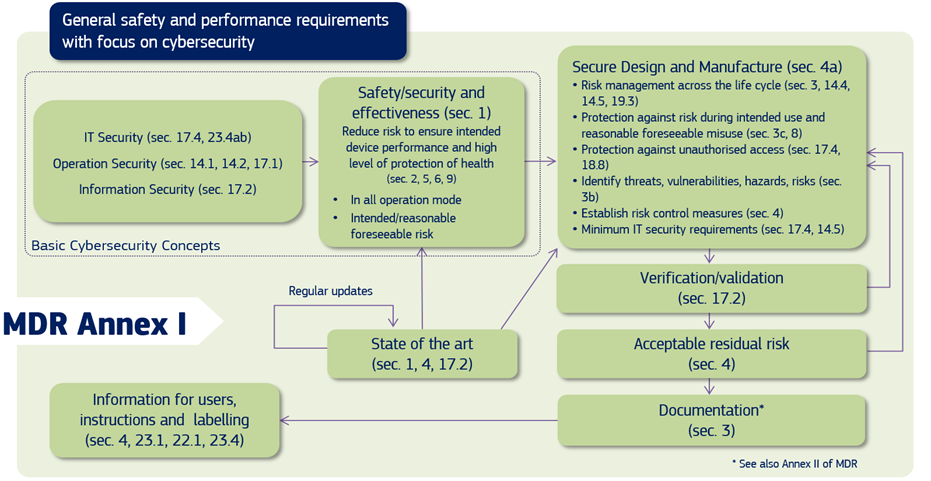
Cybersecurity For Medical Devices Omc Medical Fda cybersecurity for medical devices demands that manufacturers prioritize security from design through deployment, and even beyond. incorporating medical device penetration testing, proactive threat management, and postmarket security measures, manufacturers can ensure safety for patients as well as guarantee fda compliance, and preserve. These recommendations are intended to promote consistency, facilitate efficient premarket review, and help ensure that marketed medical devices are sufficiently resilient to cybersecurity. The u.s. food and drug administration (fda) has published new guidance on its requirement for medical device manufacturers to include details of the cybersecurity measures that have been implemented for new products in premarket submissions. As part of its secure product development framework (spdf), the fda recommends the use of the following techniques and tools to help identify and mitigate cybersecurity risks in medical devices. 1. threat modeling. threat modeling aids in identifying and addressing potential cybersecurity threats within medical device systems.
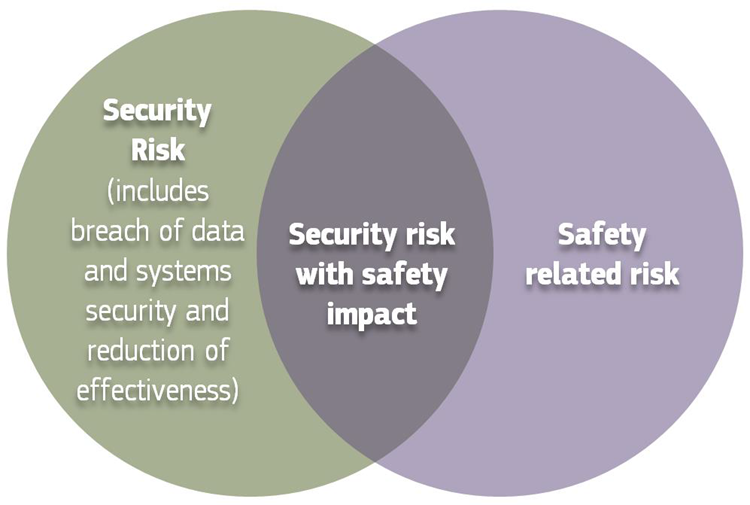
Cybersecurity For Medical Devices Omc Medical The u.s. food and drug administration (fda) has published new guidance on its requirement for medical device manufacturers to include details of the cybersecurity measures that have been implemented for new products in premarket submissions. As part of its secure product development framework (spdf), the fda recommends the use of the following techniques and tools to help identify and mitigate cybersecurity risks in medical devices. 1. threat modeling. threat modeling aids in identifying and addressing potential cybersecurity threats within medical device systems.
Cybersecurity For Medical Devices Omc Medical

Comments are closed.