First Line Therapy In The Patient With Egfr Mutant Lung Cancer

Pdf Egfr Targeted Therapy In Lung Cancer An Evolving Story Targeted treatment with egfr tyrosine kinase inhibitors (tkis) as first line therapy is a cornerstone in the treatment of egfr mutated nsclc and showed superior efficacy when compared to traditional chemotherapy, especially in individuals with specific egfr mutations. 3,4. Third generation egfr tyrosine kinase inhibitors (tkis) have become the standard of care first line treatment for advanced stage egfr mutant non small cell lung cancer (nsclc).

Egfr Exon 20 Insertions In this review, we describe the current first line treatment options for egfr mutant nsclc, provide an overview of the mechanisms of acquired resistance to third generation egfr tkis and explore novel promising treatment strategies. Multiple new therapeutic options for patients with metastatic egfr< i> mutation–positive non–small cell lung cancer have simultaneously improved patient outcomes and complicated treatment decision making. an education session at the 2025 asco annual meeting will review current data and provide guidance on frontline therapy selection. “we have now two intensified first line treatment options for patients with egfr mutant advanced nsclc, with the amivantamab lazertinib combination and the flaura 2 regimen, i.e. osimertinib and chemotherapy”, pérol concluded. A real world (rw) observational study of long term survival (lts) and treatment patterns after first line (1l) osimertinib in patients (pts) with epidermal growth factor receptor (egfr) mutation positive (m) advanced non small cell lung cancer (nsclc).

First Line Therapy In Egfr Mutant Advanced Metastatic Nsclc “we have now two intensified first line treatment options for patients with egfr mutant advanced nsclc, with the amivantamab lazertinib combination and the flaura 2 regimen, i.e. osimertinib and chemotherapy”, pérol concluded. A real world (rw) observational study of long term survival (lts) and treatment patterns after first line (1l) osimertinib in patients (pts) with epidermal growth factor receptor (egfr) mutation positive (m) advanced non small cell lung cancer (nsclc). In light of the flaura2 study results, the 2024 nccn guideline included osi pb as the new first line treatment option for egfr mutated nsclc. also, the other combination regimens involving 3g tkis are potential new first line, standard of care for this population. About egfr mutation positive non small cell lung cancer (nsclc) lung cancer remains one of the deadliest and most common cancers globally [1], with nsclc accounting for approximately 85% of cases. Osimertinib is an irreversible third generation epidermal growth factor receptor (egfr) tyrosine kinase inhibitor (tki). it is the preferred first line treatment for egfr mutated non small cell lung cancer (nsclc) compared to first generation egfr tkis. Sequential use of zorifertinib and third generation egfr tkis showed the potential to prolong patients’ survival. the results favor zorifertinib as a novel, well validated first line option for patients with egfr m nsclc and cns metastases.
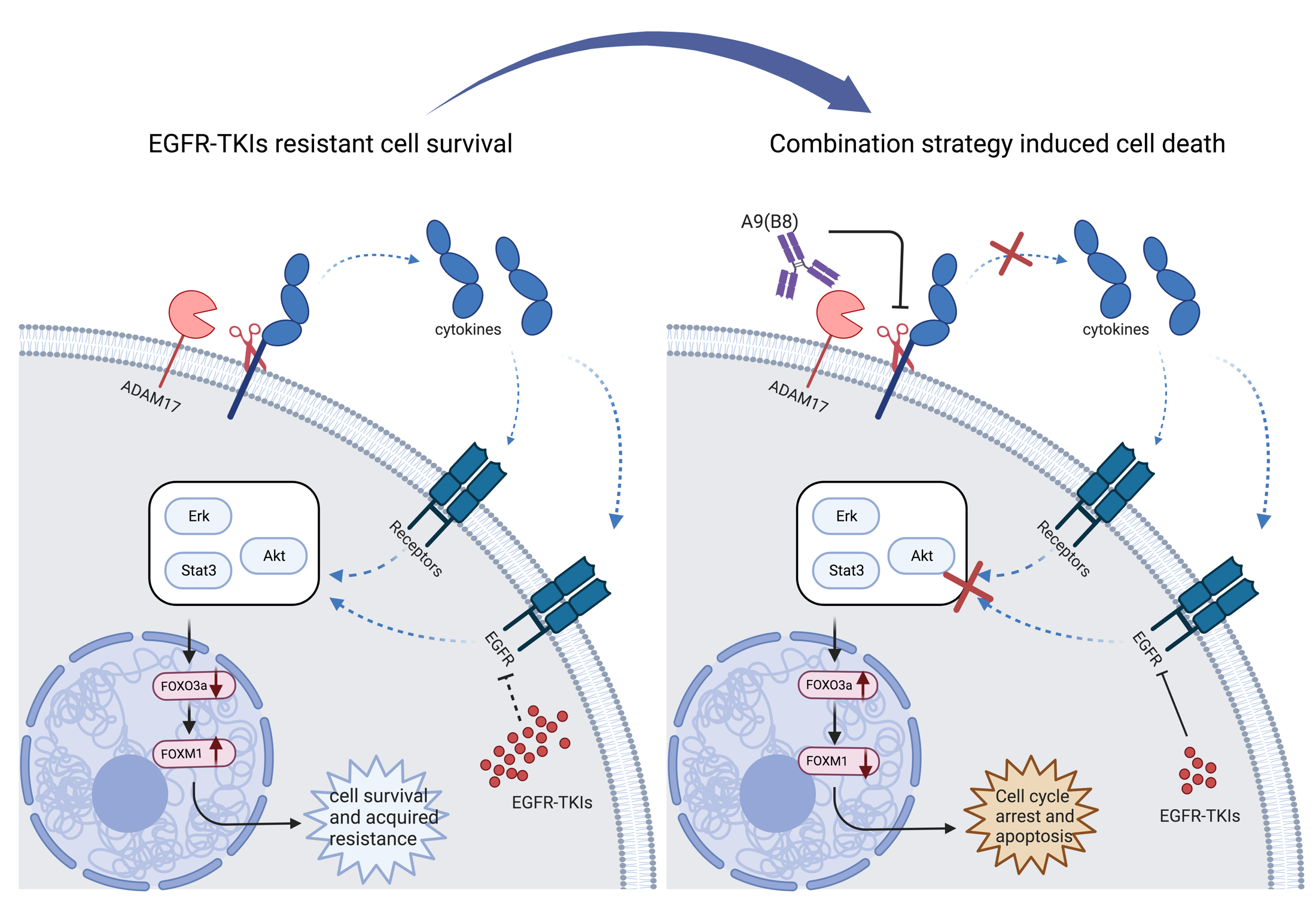
Um S New Progress In Overcoming Drug Egfr Tki Resistance In Non Small In light of the flaura2 study results, the 2024 nccn guideline included osi pb as the new first line treatment option for egfr mutated nsclc. also, the other combination regimens involving 3g tkis are potential new first line, standard of care for this population. About egfr mutation positive non small cell lung cancer (nsclc) lung cancer remains one of the deadliest and most common cancers globally [1], with nsclc accounting for approximately 85% of cases. Osimertinib is an irreversible third generation epidermal growth factor receptor (egfr) tyrosine kinase inhibitor (tki). it is the preferred first line treatment for egfr mutated non small cell lung cancer (nsclc) compared to first generation egfr tkis. Sequential use of zorifertinib and third generation egfr tkis showed the potential to prolong patients’ survival. the results favor zorifertinib as a novel, well validated first line option for patients with egfr m nsclc and cns metastases.

Comments are closed.