How To Perform A Precipitation Reaction
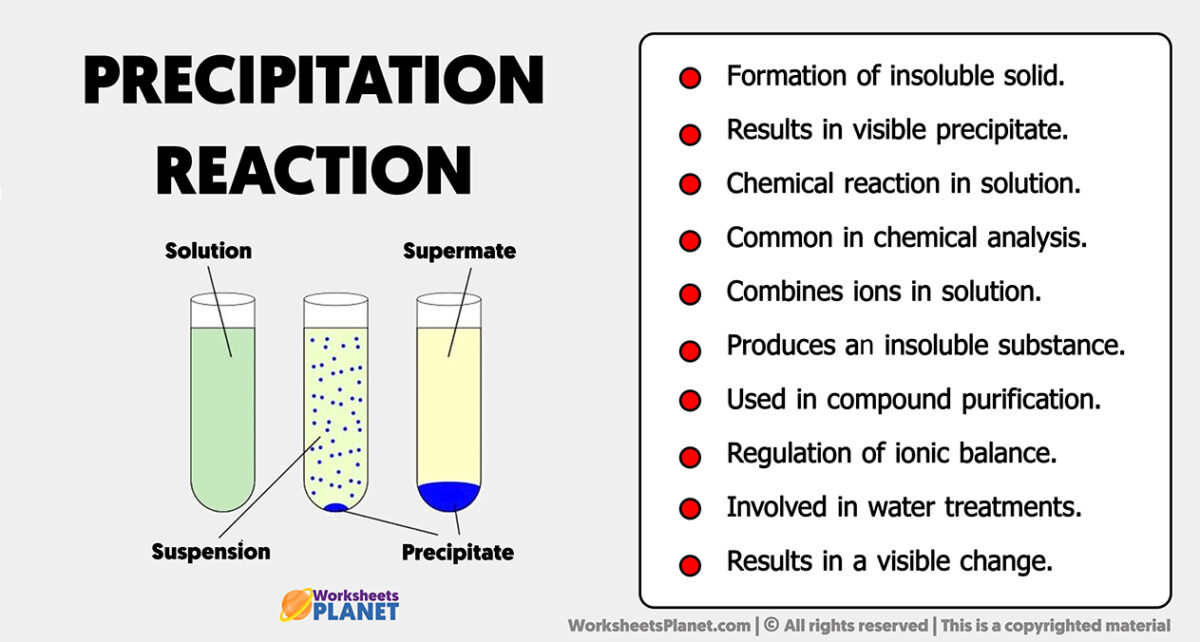
Precipitation Reaction Characteristics Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Write simple net ionic equations for precipitation reactions. a precipitation reaction is one in which dissolved substances react to form one (or more) solid products.

Precipitation Reaction Grade 10: precipitation reactions a investigate some qualitative analysis tests for certain anions (e.g. chlorides, bromides, iodides, sulfates and carbona. Video: mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{5.2.1}\)) while chemical equations show the identities of the reactants and the products and gave the stoichiometries of the reactions, but they told us very little about what was occurring in solution. A precipitation reaction forms an insoluble salt when two soluble salts combine. the resulting solution is visibly cloudy due to the formation of small aggregation of the insoluble salt. this insoluble salt falls out of the solution and is filtered to form the precipitate. How to identify a precipitation reaction. a precipitation reaction will always have a solid product. the reactants are usually two or more ionic aqueous molecules. the product must include a solid product. the most general form of a precipitation reaction then is: a (aq) b – (aq) –> ab(s) ag (aq) cl – (aq) –> agcl(s).

Precipitation Reaction A precipitation reaction forms an insoluble salt when two soluble salts combine. the resulting solution is visibly cloudy due to the formation of small aggregation of the insoluble salt. this insoluble salt falls out of the solution and is filtered to form the precipitate. How to identify a precipitation reaction. a precipitation reaction will always have a solid product. the reactants are usually two or more ionic aqueous molecules. the product must include a solid product. the most general form of a precipitation reaction then is: a (aq) b – (aq) –> ab(s) ag (aq) cl – (aq) –> agcl(s). A precipitation reaction is a reaction where aqueous solutions of soluble ionic compounds (salts), when mixed, produce an insoluble product called a precipitate. in this guide, we will learn how to predict the products of a precipitation reaction. There are three ways of representing a precipitation reaction, using a molecular equation, complete ionic equation or net ionic equation, as described in section 6.1. To identify a precipitation reaction and predict solubilities. a precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. To be able to predict the products of a double displacement reaction, you need to know the formulas and the charges of the ions in the starting materials and use the solubility rules to identify the precipitate in the reaction.

Precipitation Reaction Learn Definition Formula Reagents A precipitation reaction is a reaction where aqueous solutions of soluble ionic compounds (salts), when mixed, produce an insoluble product called a precipitate. in this guide, we will learn how to predict the products of a precipitation reaction. There are three ways of representing a precipitation reaction, using a molecular equation, complete ionic equation or net ionic equation, as described in section 6.1. To identify a precipitation reaction and predict solubilities. a precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. To be able to predict the products of a double displacement reaction, you need to know the formulas and the charges of the ions in the starting materials and use the solubility rules to identify the precipitate in the reaction.

Precipitation Reaction Examples Definition Precipitation Reaction To identify a precipitation reaction and predict solubilities. a precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. To be able to predict the products of a double displacement reaction, you need to know the formulas and the charges of the ions in the starting materials and use the solubility rules to identify the precipitate in the reaction.

Comments are closed.