Managing Stage Iii Nsclc With Actionable Egfr Or Alk Mutations

Managing Stage Iii Nsclc With Actionable Egfr Or Alk Mutations Vinay raja, md, explores the management of patients with stage iii disease and actionable egfr alk aberrations, as well as the treatment approach for patients with pd l1 <1%. Several recent trials favor molecular profile–guided precision therapy of both resectable and unresectable stage iii nsclc: highlighting the need for guideline concordant biomarker testing for all patients with stage iii nsclc.
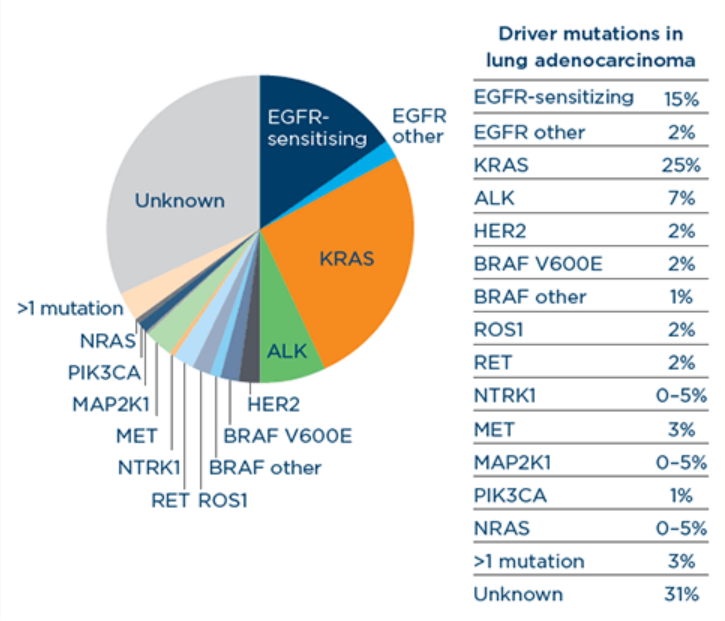
About Rare Mutations And Fusions In Nsclc Navigating Rare Mutations The phase iii randomized laura trial evaluated the third generation epidermal growth factor receptor (egfr) tyrosine kinase inhibitor osimertinib as consolidation therapy after definitive concurrent or sequential chemoradiation (crt) in 216 patients with unresectable stage iii nsclc harboring an egfr exon 19 deletion or l858r mutation. 3. For patients with resectable nsclc, two trials have demonstrated that adjuvant atezolizumab or pembrolizumab, following chemotherapy, significantly improved disease free survival (dfs). Management of stage ii nsclc . surgery: resectable stage iii patients with egfr mutations or alk re arrangements should not receive 13. tankel j, spicer j, chu q, et al. canadian consensus recommendations for the management of operable stage ii iii non small cell lung cancer: results of a modified delphi process. curr oncol. dec 06 2023. Stage iii nsclc can be classified as resectable, borderline resectable or unresectable. the aim of this review is to synthesize the latest advances for locally advanced nsclc. in the following section, the treatment options of locally advanced nsclc are discussed. to do so, the concept of resectability and treatment discussions are explored.

Pdf Outcome Of Advanced Nsclc Patients Harboring Sensitizing Egfr Management of stage ii nsclc . surgery: resectable stage iii patients with egfr mutations or alk re arrangements should not receive 13. tankel j, spicer j, chu q, et al. canadian consensus recommendations for the management of operable stage ii iii non small cell lung cancer: results of a modified delphi process. curr oncol. dec 06 2023. Stage iii nsclc can be classified as resectable, borderline resectable or unresectable. the aim of this review is to synthesize the latest advances for locally advanced nsclc. in the following section, the treatment options of locally advanced nsclc are discussed. to do so, the concept of resectability and treatment discussions are explored. In a follow up article to the updated guidelines, published in november 2024 in jco oncology practice, dr. daly and colleagues provide additional clinical insights into the management of patients with stage iii disease who do not have egfr or alk mutations. Based on data from the adaura trial, the asco panel now recommends offering adjuvant osimertinib after platinum based chemotherapy in patients with resected stage iii nsclc with egfr exon 19 deletion or exon 21 l858r mutation. Molecular diagnostics now play a pivotal role in guiding treatment strategies, with actionable genomic alterations (agas) informing the use of egfr, alk, ros1, kras, nrg1, and other targeted inhibitors in both early and advanced settings. In this trial, patients with stage ib (≥ 4 cm) to iiia nsclc harboring an alk fusion who have undergone surgical resection are randomly assigned to receive crizotinib for 2 years or observation. the primary endpoint of the trial is os, with secondary endpoints including dfs and safety profile.

Egfr And Alk Inhibitors In Nsclc Lead To Resistance Challenge In a follow up article to the updated guidelines, published in november 2024 in jco oncology practice, dr. daly and colleagues provide additional clinical insights into the management of patients with stage iii disease who do not have egfr or alk mutations. Based on data from the adaura trial, the asco panel now recommends offering adjuvant osimertinib after platinum based chemotherapy in patients with resected stage iii nsclc with egfr exon 19 deletion or exon 21 l858r mutation. Molecular diagnostics now play a pivotal role in guiding treatment strategies, with actionable genomic alterations (agas) informing the use of egfr, alk, ros1, kras, nrg1, and other targeted inhibitors in both early and advanced settings. In this trial, patients with stage ib (≥ 4 cm) to iiia nsclc harboring an alk fusion who have undergone surgical resection are randomly assigned to receive crizotinib for 2 years or observation. the primary endpoint of the trial is os, with secondary endpoints including dfs and safety profile.

Figure 2 From Systemic Treatment In Egfr Alk Nsclc Patients Second Molecular diagnostics now play a pivotal role in guiding treatment strategies, with actionable genomic alterations (agas) informing the use of egfr, alk, ros1, kras, nrg1, and other targeted inhibitors in both early and advanced settings. In this trial, patients with stage ib (≥ 4 cm) to iiia nsclc harboring an alk fusion who have undergone surgical resection are randomly assigned to receive crizotinib for 2 years or observation. the primary endpoint of the trial is os, with secondary endpoints including dfs and safety profile.

Figure 2 From Systemic Treatment In Egfr Alk Nsclc Patients Second

Comments are closed.