Ppt Aqueous Solutions Powerpoint Presentation Free Download Id 5772875

2 Aqueous Solution Chemistry Pdf Acid Aqueous Solution Aqueous solutions for example: what is the molar concentration of a naoh solution where you have been given 60 grams of naoh and ask to prepare a final volume of 3 liters?. Aqueous solutions. example the solution nacl (aq) is sodium chloride nacl (s) dissolved in water h 2 o (l) the solute is nacl (s) and the solvent is h 2 o (l). a solution is a homogeneous mixture of a solute dissolved in a solvent. the solvent is generally in excess. types of solutions. slideshow.

Aqueous Solution Pdf Aqueous Solution Applied And We are going to focus on aqueous solutions those in which the solvent is water. electrolytes and nonelectrolytes. solutions of ionic compounds and acids will conduct an electrical current. weak. – a free powerpoint ppt presentation (displayed as an html5 slide show) on powershow id: 84d8c2 otuzz. 10 water is made of very polar molecules that have a positive pole and a negative pole. since water is so polar it can dissolve most things that are polar covalent or ionic. when solutes are dissolved in water, a solution is formed. since water is the solvent, we refer to them as aqueous solutions. Aqueous solutions chemical equilibrium le châteliers principle when a stress is imposed on a system at equilibrium the system will respond in such a way as to relieve the stress. addition of a reactant change in pressure or volume of a gas change in temperature types of equilibria dissociation of water 2h2o h3o oh h2o h oh 3.

Ppt Aqueous Solutions Powerpoint Presentation Free Download Id 5401861 10 water is made of very polar molecules that have a positive pole and a negative pole. since water is so polar it can dissolve most things that are polar covalent or ionic. when solutes are dissolved in water, a solution is formed. since water is the solvent, we refer to them as aqueous solutions. Aqueous solutions chemical equilibrium le châteliers principle when a stress is imposed on a system at equilibrium the system will respond in such a way as to relieve the stress. addition of a reactant change in pressure or volume of a gas change in temperature types of equilibria dissociation of water 2h2o h3o oh h2o h oh 3. Electricity is moving charges. the ions that are dissolved can move. solutions of ionic compounds can conduct electricity. electrolytes. solutions are classified three ways. 8 types of solutions. Aqueous solutions. example the solution nacl (aq) is sodium chloride nacl (s) dissolved in water h 2 o (l) the solute is nacl (s) and the solvent is h 2 o (l). a solution is a homogeneous mixture of a solute dissolved in a solvent. in aqueous solutions solvent is water. Aqueous solutions. write what is in the purple boxes. ionic equations. when a soluble substance is dissolved in water, the substance often breaks into ions. this solution is said to be an aqueous solution. pb (no 3 ) 2 (aq) pb 2 (aq) 2no 3 (aq) nai (aq) na (aq) i (aq). Reactions in aqueous solutions • aqueous solution: • solution in which water is the solvent (dissolving agent). • 3 major types of chemical processes of aqueous solutions: • precipitation reactions • acid base reactions • redox reactions.

Ppt Aqueous Solutions Powerpoint Presentation Free Download Id 9325484 Electricity is moving charges. the ions that are dissolved can move. solutions of ionic compounds can conduct electricity. electrolytes. solutions are classified three ways. 8 types of solutions. Aqueous solutions. example the solution nacl (aq) is sodium chloride nacl (s) dissolved in water h 2 o (l) the solute is nacl (s) and the solvent is h 2 o (l). a solution is a homogeneous mixture of a solute dissolved in a solvent. in aqueous solutions solvent is water. Aqueous solutions. write what is in the purple boxes. ionic equations. when a soluble substance is dissolved in water, the substance often breaks into ions. this solution is said to be an aqueous solution. pb (no 3 ) 2 (aq) pb 2 (aq) 2no 3 (aq) nai (aq) na (aq) i (aq). Reactions in aqueous solutions • aqueous solution: • solution in which water is the solvent (dissolving agent). • 3 major types of chemical processes of aqueous solutions: • precipitation reactions • acid base reactions • redox reactions.
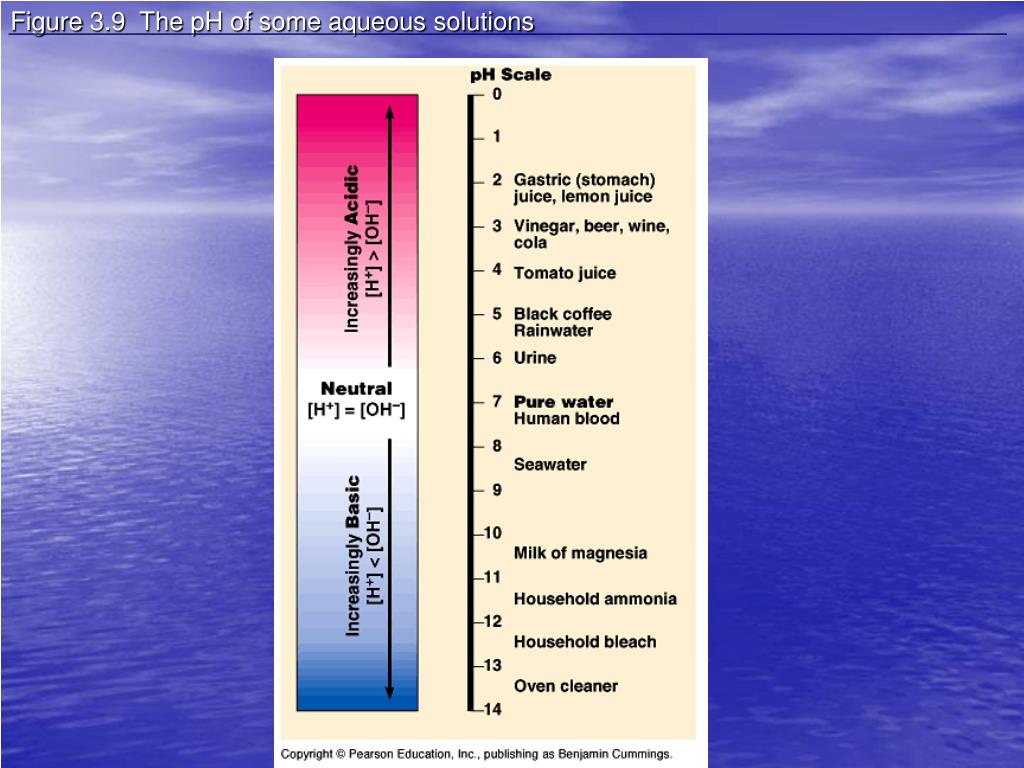
Ppt Aqueous Solutions Powerpoint Presentation Free Download Id 5772875 Aqueous solutions. write what is in the purple boxes. ionic equations. when a soluble substance is dissolved in water, the substance often breaks into ions. this solution is said to be an aqueous solution. pb (no 3 ) 2 (aq) pb 2 (aq) 2no 3 (aq) nai (aq) na (aq) i (aq). Reactions in aqueous solutions • aqueous solution: • solution in which water is the solvent (dissolving agent). • 3 major types of chemical processes of aqueous solutions: • precipitation reactions • acid base reactions • redox reactions.

Comments are closed.