Precipitation Reaction Definition And Examples In Chemistry

Precipitation Reaction Definition And Examples In Chemistry In chemistry, a precipitation reaction is a chemical reaction between two dissolved substances that forms one or more solid products. the solid is the precipitate. the remaining solution is the supernate or supernatant. there are two common ways to indicate precipitation in a chemical reaction. Precipitation reaction is an chemical reaction occurring in aqueous solutions where two ionic bonds combine forming up insoluble salts. learn more about the definition, examples & equations of precipitation reaction.

Precipitation Reaction Definition Examples And Uses Vrogue Co Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. What is precipitation reaction? a precipitation reaction forms an insoluble salt when two soluble salts combine. the resulting solution is visibly cloudy due to the formation of small aggregation of the insoluble salt. this insoluble salt falls out of the solution and is filtered to form the precipitate. What are precipitation reactions? – one common type of reaction that occurs in an aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement , double replacement , or metathesis reactions.

60 Examples Of Precipitation Reaction Vivid Examples What are precipitation reactions? – one common type of reaction that occurs in an aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement , double replacement , or metathesis reactions. A precipitation reaction involves the formation of an insoluble compound during a chemical reaction between soluble reactants. for example, when silver nitrate (agno₃) combines with sodium chloride (nacl), you get silver chloride (agcl) as a precipitate. Video: mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{5.2.1}\)) while chemical equations show the identities of the reactants and the products and gave the stoichiometries of the reactions, but they told us very little about what was occurring in solution. Precipitation reactions are useful to identify ions and prepare specific compounds. a precipitation reaction is a type of chemical reaction in which two soluble salts in aqueous solution combine and one of the products is an insoluble salt called a precipitate. Precipitation reactions are an example of double displacement reactions in which a solid form residue called the precipitate is left behind. when two or more two solutions containing different salts are combined, insoluble salts are produced that precipitate out of the solution.

Precipitation Reaction Examples Definition Precipitation Reaction A precipitation reaction involves the formation of an insoluble compound during a chemical reaction between soluble reactants. for example, when silver nitrate (agno₃) combines with sodium chloride (nacl), you get silver chloride (agcl) as a precipitate. Video: mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{5.2.1}\)) while chemical equations show the identities of the reactants and the products and gave the stoichiometries of the reactions, but they told us very little about what was occurring in solution. Precipitation reactions are useful to identify ions and prepare specific compounds. a precipitation reaction is a type of chemical reaction in which two soluble salts in aqueous solution combine and one of the products is an insoluble salt called a precipitate. Precipitation reactions are an example of double displacement reactions in which a solid form residue called the precipitate is left behind. when two or more two solutions containing different salts are combined, insoluble salts are produced that precipitate out of the solution.
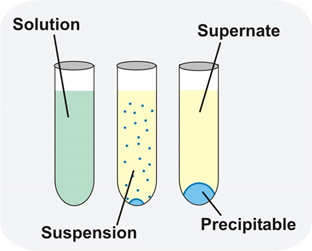
Precipitation Reaction In Chemistry Precipitation Reaction Equation Precipitation reactions are useful to identify ions and prepare specific compounds. a precipitation reaction is a type of chemical reaction in which two soluble salts in aqueous solution combine and one of the products is an insoluble salt called a precipitate. Precipitation reactions are an example of double displacement reactions in which a solid form residue called the precipitate is left behind. when two or more two solutions containing different salts are combined, insoluble salts are produced that precipitate out of the solution.

Precipitation Reaction Insights Everyday Uses

Comments are closed.