Precipitation Reaction Learn Definition Formula Reagents

Precipitation Reaction Insights Everyday Uses Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Know about precipitation reaction, definition, formula, change reagents used like potassium chloride and silver nitrate, properties, types, applications.
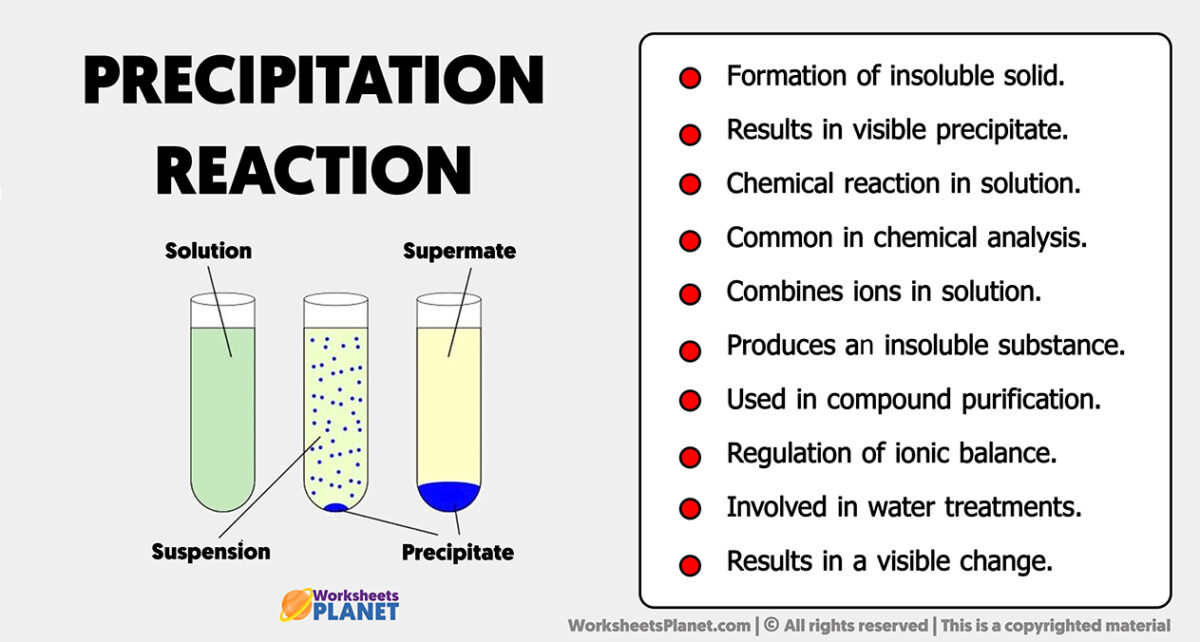
Precipitation Reaction Characteristics In chemistry, a precipitation reaction is a chemical reaction between two dissolved substances that forms one or more solid products. the solid is the precipitate. the remaining solution is the supernate or supernatant. there are two common ways to indicate precipitation in a chemical reaction. Video: mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{5.2.1}\)) while chemical equations show the identities of the reactants and the products and gave the stoichiometries of the reactions, but they told us very little about what was occurring in solution. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions. Precipitation is one type of chemical reaction which involves the formation of one or more insoluble products. precipitation reactions, also called double displacement reactions can be summarized with the following reaction equation: ab (a q) cd (a q) ad (s) cb (a q) o r (s).

Precipitation Reaction Definition Examples And Uses Vrogue Co A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions. Precipitation is one type of chemical reaction which involves the formation of one or more insoluble products. precipitation reactions, also called double displacement reactions can be summarized with the following reaction equation: ab (a q) cd (a q) ad (s) cb (a q) o r (s). What is precipitation reaction? precipitate meaning in chemistry precipitation reaction definition: the word precipitation meaning refers to a chemical reaction formation that occurs in an aqueous solution and results in the creation of an insoluble salt when two ionic bonds join. – one common type of reaction that occurs in an aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate. – a precipitate is an insoluble solid that separates from the solution. – precipitation reactions usually involve ionic compounds. A precipitation reaction is a type of chemical reaction that occurs when two soluble salts react in solution to form an insoluble product, known as a precipitate. this reaction is significant in identifying the formation of new compounds and plays an important role in various chemical processes, including qualitative analysis and purification. To identify a precipitation reaction and predict solubilities. a precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed.

Pleasing Precipitation Performances The Microscale Way Science What is precipitation reaction? precipitate meaning in chemistry precipitation reaction definition: the word precipitation meaning refers to a chemical reaction formation that occurs in an aqueous solution and results in the creation of an insoluble salt when two ionic bonds join. – one common type of reaction that occurs in an aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate. – a precipitate is an insoluble solid that separates from the solution. – precipitation reactions usually involve ionic compounds. A precipitation reaction is a type of chemical reaction that occurs when two soluble salts react in solution to form an insoluble product, known as a precipitate. this reaction is significant in identifying the formation of new compounds and plays an important role in various chemical processes, including qualitative analysis and purification. To identify a precipitation reaction and predict solubilities. a precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed.

Precipitation Reaction Learn Definition Formula Reagents A precipitation reaction is a type of chemical reaction that occurs when two soluble salts react in solution to form an insoluble product, known as a precipitate. this reaction is significant in identifying the formation of new compounds and plays an important role in various chemical processes, including qualitative analysis and purification. To identify a precipitation reaction and predict solubilities. a precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed.

Comments are closed.