Predicting Precipitation Reactions Pdf Name Regents Chemistry

Regents Chemistry Hw Pdf Gases Physical Sciences Name: regents chemistry predicting precipitation reactions directions: to predict whether a precipitation reaction will take place between two aqueous solutions and if the answer is yes, to write the complete equation for the reaction. ex. li 2 co 3 (aq) al(no 3) 3. Name: regents chemistry predicting precipitation reactions directions: to predict whether a precipitation reaction will take place between two aqueous solutions and if the answer is yes, to write the complete equation for the reaction. ex. li 2 co 3 (aq) al(no 3) 3 (aq) ?.
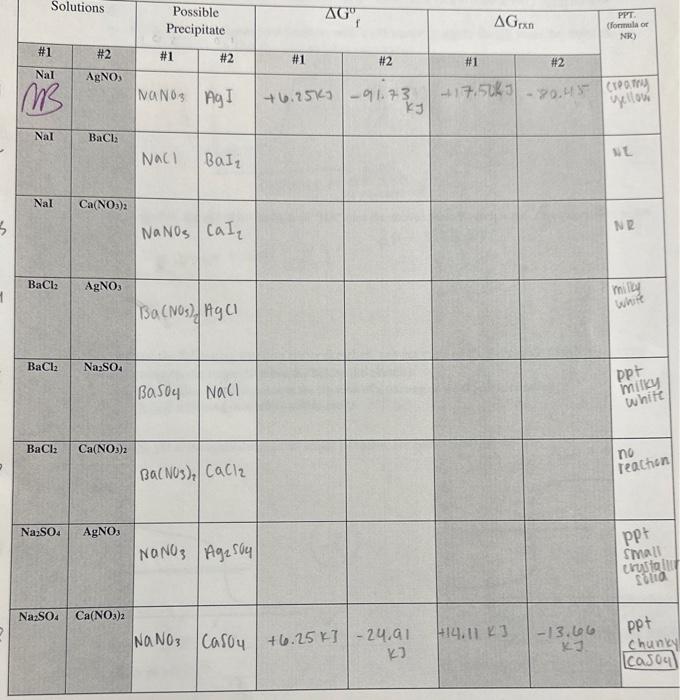
Solved Predicting Precipitation Reactions Chegg Thermodynamic calculations allow us to calculate and predict whether a reaction will be spontaneous. in order to understand how we can perform such calculations and make a conclusion about spontaneity, we need to first understand a few thermodynamic functions. Mixing of dissolved ionic compounds can lead to a precipitation reaction, an example of a double displacement reaction. the precipitate is a solid product, a new ionic compound that is different from the reactants in both composition and solubility. When mercury(i) nitrate and potassium phosphate solutions are mixed, mercury(i) phosphate precipitates. write the net ionic equation. predict the outcome of the mixing of silver nitrate and potassium carbonate solutions. Describe precipitation reactions by writing net ionic equations. understand the relationship between solubility and precipitation reactions. ionic compounds in solution consist of free ions surrounded by water.

Precipitation Reactions When mercury(i) nitrate and potassium phosphate solutions are mixed, mercury(i) phosphate precipitates. write the net ionic equation. predict the outcome of the mixing of silver nitrate and potassium carbonate solutions. Describe precipitation reactions by writing net ionic equations. understand the relationship between solubility and precipitation reactions. ionic compounds in solution consist of free ions surrounded by water. Predicting k2so4(aq) pb(no3)2(aq) = ? ? 1. identify the ions that are in the original solutions: 2. swap them and write the write resultant compounds as possible products: 3. is one or both of the new salts insoluble solubility guidelines. 4. now write and balance the chemical equation:. In this lab you will use your knowledge of precipitation tables to predict precipitation reactions. examine the lists of solutions you will be using for this experiment. you will be mixing solutions from set a with set b. which combinations do you predict will result in a precipitate? record your predictions. Precipitation reactions: a chemical reaction that involves the formation of an insoluble product (precipitate; solid) is called precipitation reaction. the reactants are soluble, but the product formed would be insoluble and separates out as a solid. You will learn how to identify the formation of a precipitate, predict the products of a reaction based on solubility rules, and calculate the mass of the precipitate formed. this experiment revolves around the reaction between aqueous solutions of different salts.

Comments are closed.