Solution Empirical Formula Molecular Formula Determination From

Empirical Formula Determination | PDF | Mole (Unit) | Molecules
Empirical Formula Determination | PDF | Mole (Unit) | Molecules The chemical formula for a compound obtained by composition analysis is always the empirical formula. we can obtain the chemical formula from the empirical formula if we know the molecular weight of the compound. Learn how to find a compound’s empirical and molecular formulas using mass or percent composition data, molar mass, and example calculations.

SOLUTION: Empirical Formula Molecular Formula Determination From ...
SOLUTION: Empirical Formula Molecular Formula Determination From ... Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. as the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. Determining the molecular formula from the provided data will require comparison of the compound’s empirical formula mass to its molar mass. as the first step, use the percent composition to derive the compound’s empirical formula. Determining the molecular formula from the provided data will require comparison of the compound’s empirical formula mass to its molar mass. as the first step, use the percent composition to derive the compound’s empirical formula. Given the chemical formula of the substance, we were able to determine the amount of the substance (moles) from its mass, and vice versa. but what if the chemical formula of a substance is unknown?.

SOLUTION: Empirical Formula And Molecular Formula Complete Chemistry ...
SOLUTION: Empirical Formula And Molecular Formula Complete Chemistry ... Determining the molecular formula from the provided data will require comparison of the compound’s empirical formula mass to its molar mass. as the first step, use the percent composition to derive the compound’s empirical formula. Given the chemical formula of the substance, we were able to determine the amount of the substance (moles) from its mass, and vice versa. but what if the chemical formula of a substance is unknown?. Given the chemical formula of the substance, we were able to determine the amount of the substance (moles) from its mass, and vice versa. but what if the chemical formula of a substance is unknown?. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. as the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. as the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. The empirical formula of a substance can be calculated from its percent composition, and the molecular formula can be determined from the empirical formula and the compound’s molar mass.

Empirical And Molecular Formula Notes
Empirical And Molecular Formula Notes Given the chemical formula of the substance, we were able to determine the amount of the substance (moles) from its mass, and vice versa. but what if the chemical formula of a substance is unknown?. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. as the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. as the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. The empirical formula of a substance can be calculated from its percent composition, and the molecular formula can be determined from the empirical formula and the compound’s molar mass.
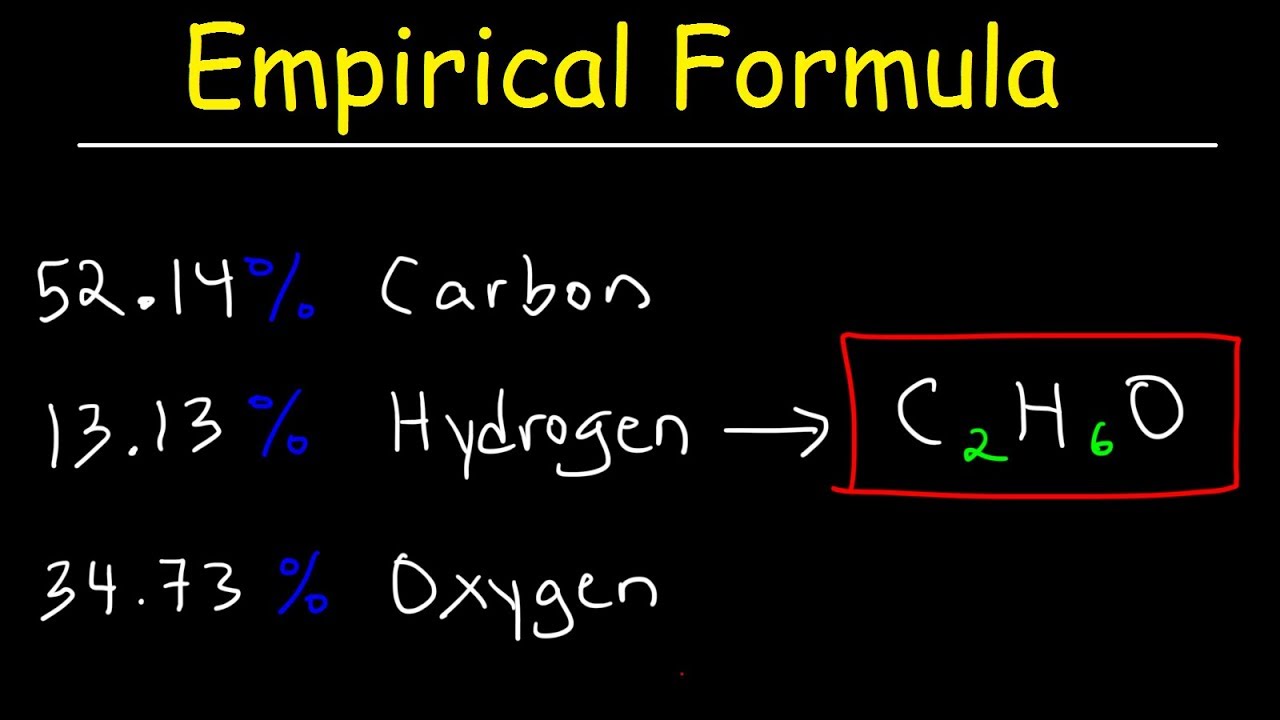
Empirical Formula & Molecular Formula Determination From Percent Composition
Empirical Formula & Molecular Formula Determination From Percent Composition
Related image with solution empirical formula molecular formula determination from
Related image with solution empirical formula molecular formula determination from
About "Solution Empirical Formula Molecular Formula Determination From"

















Comments are closed.