What Are The 3 Steps To Determining An Empirical Formula Given The

Determining Empirical Formula Steps By Firestarter Tpt That’s where the empirical formula comes in! whether you’re a chemistry student, a curious learner, or someone brushing up on basics, this guide will make it easy to understand what an empirical formula is and how to calculate it—step by step. Steps to determine empirical formula: assume a 100g 100 g sample of the compound so that the given percentages can be directly converted into grams. use each element's molar mass to convert the grams of each element to moles.

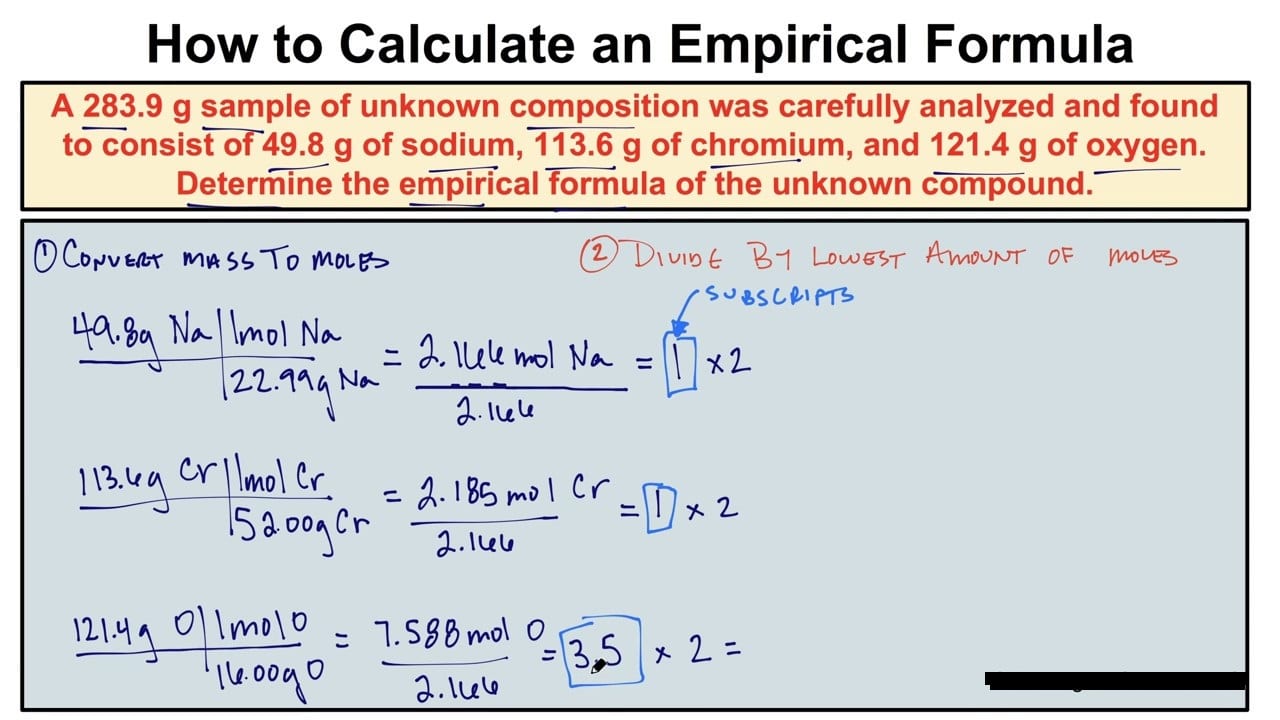
Steps For Determining An Empirical Formula Lecture Notes Chemistry Step 1: determine the masses. step 2: determine the number of moles by dividing the grams by the atomic mass. step 3: divide the number of moles of each element by the smallest number of moles. how do you find empirical formula from percent composition? can you determine the molecular formula from its percent composition?. You should be able to determine the empirical formula for any compound as long as you know the mass of each element present, the percentage of mass for each present element, or the molecular formula of the compound. Step 1: assume you have 100 grams of the sample. this makes it easier to determine the amounts in grams for each element in the compound from the given % compositions. step 2: convert these weights into moles. use a periodic table to get the atomic weights of each element. step 3: determine the smallest whole number ratio between these values. The steps involved in the process of determining empirical formula is following: obtain the percentage composition of elements in the compound, either from experimental data or by converting mass data to percentages.

Simplychemistry Steps To Solve Empirical Formula Step 1: assume you have 100 grams of the sample. this makes it easier to determine the amounts in grams for each element in the compound from the given % compositions. step 2: convert these weights into moles. use a periodic table to get the atomic weights of each element. step 3: determine the smallest whole number ratio between these values. The steps involved in the process of determining empirical formula is following: obtain the percentage composition of elements in the compound, either from experimental data or by converting mass data to percentages. This lecture is about how to calculate empirical formula in 3 easy steps.following are the three easy steps to calculate the empirical formula of any compoun. Step 1: list the known quantities and plan the problem. empirical formula = fe? o? steps to follow are outlined in the text. step 2: calculate. 1. assume a 100 g sample. @$$\begin {align*}& 69.94 \ \text {g fe} \\ & 30.06 \ \text {g o}\end {align*}@$$ 2. convert to moles. Determining empirical formulas helps in identifying unknown compounds, analyzing reaction ratios, and understanding molecular composition in chemical studies. 1. obtain mass or percentage composition. the mass or percentage of each element in a compound is required. 2. convert mass to moles. 3. determine the simplest ratio.
Empirical Formula Definition Calculator Best Examples Get Education Bee This lecture is about how to calculate empirical formula in 3 easy steps.following are the three easy steps to calculate the empirical formula of any compoun. Step 1: list the known quantities and plan the problem. empirical formula = fe? o? steps to follow are outlined in the text. step 2: calculate. 1. assume a 100 g sample. @$$\begin {align*}& 69.94 \ \text {g fe} \\ & 30.06 \ \text {g o}\end {align*}@$$ 2. convert to moles. Determining empirical formulas helps in identifying unknown compounds, analyzing reaction ratios, and understanding molecular composition in chemical studies. 1. obtain mass or percentage composition. the mass or percentage of each element in a compound is required. 2. convert mass to moles. 3. determine the simplest ratio.
.PNG)
Find Empirical Formula From Equation Tessshebaylo Determining empirical formulas helps in identifying unknown compounds, analyzing reaction ratios, and understanding molecular composition in chemical studies. 1. obtain mass or percentage composition. the mass or percentage of each element in a compound is required. 2. convert mass to moles. 3. determine the simplest ratio.

Find Empirical Formula From Equation Tessshebaylo

Comments are closed.